New surgical products instrumental to enhancing OR experience
As long as the demand for more complex surgical procedures continues to grow alongside emerging research, medical device manufacturers will continue to develop the products that surgeons need to perform them.
“New products and innovations are being developed all the time and facilities and their healthcare workers have a responsibility to assess these new technologies and techniques,” said Pam Werner, MBA, BSN, RN, CNOR, Sr. Clinical Consultant, Ansell. “They need to ask suppliers what is new and how it will be of value in their facility.”
Traditional devices are also getting redesigned or upgraded to satisfy surgery’s changing needs. Surgeons can expect to find enhanced safety features, improved ergonomics, better precision, computer-assisted features, visual technology, robotic aides and more. Here’s a brief roundup of some of the latest products surgeons of various specialties are using in the OR.
Cutting edge blades
Marilyn Norrie, Ansell’s Senior Specialist for Brand/Product Marketing, says Ansell’s SANDEL branded products offer clinicians a variety of sharps safety devices that are designed to improve compliance among end-users. “Many healthcare facilities have embraced innovations in sharps safety and seen reductions in sharps injuries as a result,” said Norrie. “Facilities with high levels of engagement in sharps safety across the healthcare team tend to see improvements in sharps injury rates.”
Werner described one of the products: ”CHANGE-A-BLADE Safety Scalpel Handle is a disposable safety scalpel handle designed to accept any manufacturer’s stainless steel or carbon blades. The blade can be changed at any time during the procedure. CHANGE-A-BLADE is weighted to mimic a traditional scalpel. It offers full visibility for the surgeon to see what is being cut and is available in different sizes.”
MYCO Medical’s new Technocut Brand Premium Surgical Blade Line, launched in September, is designed to give surgeons what they want: super-sharp, durable blades with lasting edges, smooth tissue incision capability and reduced drag.
“The Technocut Brand Premium Surgical Blade line is produced using a premium grade of steel and the cutting edge undergoes a proprietary process that reduces friction,” said Sam Kumar, President, CEO. “This may result in fewer blades being required during a procedure, which may reduce the risk associated with blade changeouts during a procedure.”
Kumar added that the blades are “typically 25 percent to 35 percent lower [in cost] than the leading national brand, making them a tremendous value.”
Tapped for spinal surgery
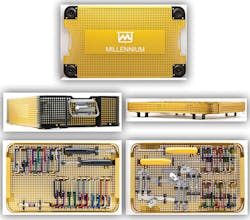
Millennium Surgical’s new lumbar and cervical spine sets were launched late last year and serve as a cost-effective alternative to replacing parts or purchasing new sets. “Replacing existing Shadow-Line and TrimLine sets and individual parts is cost-prohibitive for many facilities,” said Millennium President Robert Edelstein. “We offer complete and scaled-down sets, along with blades and components that are compatible with both systems at hundreds of dollars less. And we can help facilities further reduce costs by accepting existing sets as trade-in.”
Unlike traditional blades, Edelstein said Millennium blades are manufactured with aircraft-grade 60/61 aluminum, with alignment grooves in the blade posts to support proper locking of the retractor frames and handles and lessen the chance of accidental blade release. “Some traditional frames feature a locking button on the top of the retractor arm, which can easily be bumped by Kerrisons or other instruments. The Quick Release Handles feature a thumb release, eliminating torqueing when unscrewing a blade,” Edelstein explained, adding that traditional replacement parts and handles are still available for those who prefer them.
Marilyn M. Burns RN, BS, CNOR, Director of Clinical Affairs and Medical Education, Symmetry Surgical, shared information about the company’s single-use Kerrison product, which was introduced in 2014 and developed based on surgeons’ desire to have consistently sharp tips during procedures. “The Symmetry Sharp Kerrison is a first-of-its-kind, patented Kerrison that provides a new tip every time — with 21 possible handle and tip configurations,” explained Burns. “A sharp Kerrison tip provides precise cutting of bony tissue of the spine and reduces surgeon hand fatigue, which potentially leads to safer and more precise neurospine procedures.”
Based on user feedback, Symmetry added a new thin footplate tip last year to give surgeons better access to tight cervical spaces. “Symmetry Sharp Kerrisons combine the value of a reusable instrument with the precision of a sharp cutting edge whenever it’s needed,” said Burns. “Repair costs for sharpening or replacing traditional Kerrison instruments with dull tips are alleviated by the single-use Symmetry Sharp Kerrison tips.”
Inside track on laproscopes
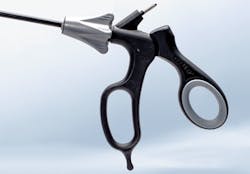
Ryan Mancini, Senior Product Manager, Aesculap, Inc., says the company expects a February launch of its new laparoscopic handles which are designed to work with Aesculap’s four piece, take-apart laparoscopic instruments. The new handles are engineered specifically to give surgeons and sterile processing professionals added comfort and convenience.
“Surgeons, and hospitals in general, may have to compromise between clinical performance and surgeon comfort in the OR, and the ability to efficiently and effectively reprocess instruments in the central sterile department,” said Mancini. “The new laparoscopic handles provide surgeons increased options to help meet their ergonomic needs through a more convenient ratchet toggle placement and increased comfort in the front and rear rings of the handle.”
The handles will also help to reduce instrument-induced fatigue among surgeons with heavy case loads and it gives sterile processing staff an easier way to inspect the lumens during decontamination. “The complete instrument is validated for sterilization while fully assembled, which means it does not require any assembly by OR staff. This reduces setup time and allows OR staff to focus on other pre-operative tasks,” Mancini added. “Aesculap’s four-piece, take-apart design not only provides optimal cleaning but, if a component wears out, it also allows the hospital to repair or replace a single part, not the entire instrument. This helps to eliminate costly reinsulation repair costs and helps to minimize the frequency of sets being taken out of rotation due to instrument repairs.”
Speaking of insulation, did you know that a significant number of laparoscopic instruments may not be safe to use on patients? That’s according to Lisa Hawley, Product Manager, Mobile Instrument Service & Repair, Inc. who said her company discovered as much as “43 percent of all laparoscopic devices currently in use have a known defect which has a potential to cause accidental patient burns.” Even just a tiny pinhole in the insulation of an instrument, including single-use devices, is enough of an opening to allow an electric current to escape and seriously damage neighboring tissue. “Patients that endure a burn have a very long road to recovery which impacts facilities at multiple levels,” said Hawley.
Mobile Instrument’s InsulScan is an electrosurgical instrument insulation tester that identifies dangerous defects before the device even reaches the surgeon’s hand. “Facilities did not have a way to effectively test in the OR before and immediately following laparoscopic procedures,” said Hawley. “With the increase of laparoscopic instruments being used for different types of surgical procedures, instruments not being checked correctly run a greater risk of hurting a patient. InsulScan reduces liability exposure for the surgeons and hospital. When used as recommended in the operating room InsulScan reduces the risk of patient burn to near zero.”
AORN and ECRI recommend that facilities test all active electrodes prior to and after surgery. Hawley says as of now, the only known device that can do this in the sterile field is InsulScan. Mobile Instrument also has a Laparoscopic Burn study guide for nurses and sterile processing professionals.
Orthopedic optimization
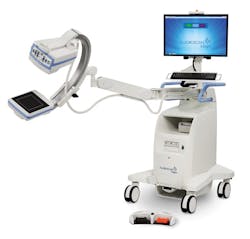
Hologic, Inc.’s next generation in mini C-arm imaging, the Fluoroscan InSight FD Mini C-Arm, just launched in late January to help clinicians improve skeletal healthcare. The upgrade gives orthopedists, podiatrists and clinicians diversified imaging options, more flexible storage and transport, and an enhanced interface. Hologic also streamlined the monitor arm with an integrated keyboard and reduced top casting for increased range of motion and positioning options in the OR; and it has a simpler C-arm locking mechanism, reducing the system’s total width when locked.
“These enhancements address major pain points and offer solutions that increase workflow and productivity, ultimately leading to a better patient experience,” said Pete Valenti, Hologic’s Division President, Breast and Skeletal Health Solutions. “The Fluoroscan InSight FD Mini C-arm provides high-resolution and low-dose rate modes, which deliver the largest image size and highest image resolution available, on an intuitive 24-inch HD touchscreen. The low-dose rate mode allows physicians to reduce dose rates by up to 50 percent compared to Auto Mode, while continuing to deliver clinically equivalent images. The high-resolution mode enables clinicians to use full detector resolution.”
Other features include the MegaView Image which increases image size by 50 percent during the Review Mode, a pinch-to-zoom feature, save notifications for identifying saved images, a save filter to let the clinician filter for saved images, and an auto-save option to save all images acquired during a session. Current Fluoroscan InSight FD Mini C-Arm customers can access the system’s software upgrades.
Also new in spine surgery devices is the Table Top Rod Shears launched last year by gSource, for sturdy, accurate rod-shearing and improved patient safety features.
“The table top design allows the surgeon to cut on a sturdy surface and gives the surgeon more leverage in order to easily cut rods. The handle extends and locks to provide surgeons with increased leverage, allowing for greater ease in cutting rods,” said Liz Ostrow, Marketing Manager. “It also helps prevent the cut piece of rod from coming into contact with the patient or falling into the surgical site. Rods are sheared, leaving a smooth and clean surface rather than a sharp, jagged, burr-like surface common when using a standard pinching-type rod cutter. This results in less trauma for the patient. ”
The device also increases efficiency by offering two different rod diameters — 5.0mm [3/16”] and 6.35mm [1/4”] — with rod diameter holes clearly marked and a collapsible handle for easier storage.
Single-use gets eco-friendly
For surgical facilities seeking environmentally preferable products (EPPs) for the OR, NewGen Surgical makes redesigned single-use surgical products for delivering sustainable, quality patient care. The NewGen Surgical Skin Stapler is a lightweight single-use device made from 69 percent plant-based material, reducing the energy used during production by 67 percent compared to plastic disposables.
“Using NewGen Surgical products is not a one-time benefit, but a measurable avoidance of plastic waste — for each product — year after year,” said Rob Chase, Founder and President of NewGen Surgical. “Really, it’s a way forward out of our dependence on single-use disposable plastic products. While the medical device market is a complicated space, after a period of development to ensure efficacy, performance and regulatory compliance, our products can help customers looking for healthier product solutions. Just one healthcare system could avoid contributing several tons of plastic waste generated from their OR by switching to the same product made from sustainable material. That’s one system, one product.”
The stapler holds 35 stainless steel staples and comes with a visible staple-remaining indicator. The ergonomically designed device also accommodates different hand sizes and grips, and features a low-force firing cycle for ease of use. Other features include an alignment indicator for accurate staple placement, quality assurance testing, and it is made without latex.
Carol Summers, Senior Marketing Manager, says the product’s clinical performance is similar to other skin staplers on the market for routine skin closure. “Driven by performance, economic value and sustainability, our products are priced competitively and perform the same function in the same way,” Summers explained. “NewGen Surgical products are differentiated through sustainability. Every piece of plastic matters and there is opportunity to replace existing products with an option that has less of an environmental impact without disrupting the current workflow of a surgical department.”
2018 Surgical Instrument & Accessory Vendors & Product Spotlights

Valerie J. Dimond | Managing Editor
Valerie J. Dimond was previously Managing Editor of Healthcare Purchasing News.