What are some of the most obscure warning signs your endoscopic equipment has not been cleaned properly before high-level disinfection or sterilization? Sterile processing experts from providers and suppliers highlight missing links from the fundamental to the advanced.
A warning sign that is less obvious may be repeated failure of a cleaning verification test. However, there are additional factors that can contribute to an improperly cleaned device. For instance, damage to endoscope channels, poor quality or contaminated water, contaminated AER, and cleaning accessories that are reused without disinfection between uses can be detrimental to proper cleaning.
Janet Prust, Director, Global Scientific Marketing and Education, 3M Health Care
Besides the obvious warning signs, there are more obscure signs that may point out potential warnings for improperly cleaned scopes, such as routine delays in reprocessing. The relative guidance tells us that endoscopes should not sit for more than one hour after the procedure has finished. Staff should be observed to see if they know and understand the difference between cleaning, disinfection and sterilization, and that they are following nationally accepted guidelines for reprocessing endoscopes. Lack of quality measures or documentation confirming steps of reprocessing may be a warning sign. There are warning signs within the facility as well, such as access to only one sink and Chux pads present underneath endoscopes in the storage closet. A dripping scope is a sign that it has not been cleaned properly.
Grace Thornhill, Ph.D., Infection Prevention Fellow, Boston Scientific Corp.
- Manual cleaning of endoscope channels not performed correctly. The inner lumens of endoscopes are not visible without the assistance of specialized tools. To best mitigate this matter, the manual cleaning procedure outlined in the endoscope manufacturer’s IFU must be followed exactly.
- Incompatibility of reprocessing accessories, such as the use of incorrect channel brushes (i.e., wrong diameter or length) or incorrect cleaning adaptors. Refer to the endoscope manufacturer’s IFU regularly to ensure correct accessories are used by all staff.
- Endoscope transport to reprocessing area is delayed, which may not be obvious to the reprocessing technician. Endoscopes that are not reprocessed in a timely manner may need to undergo additional cleaning steps if the endoscope manufacturer requires it.
- Reprocessing steps are skipped, which may not be obvious if multiple reprocessing technicians are working in the same area. Staff must ensure that all steps are performed completely and correctly.
- Pre-cleaning not performed, or preformed incorrectly, which may not be obvious to technicians working in the reprocessing area. Pre-cleaning must be performed after each endoscopy case and is an important step for removing bioburden and preventing the formation of biofilm
- Documentation incomplete, which may not be obvious with a quick glance at the paper receipts.
- Discolored drippings in the storage cabinet, which may indicate improper rinsing or cleaning.
Melinda Benedict, CFER, Manager, Infection Control Program, Olympus America Inc.
It is often the less-obvious indications that enable identification of improper cleaning practices. Among these subtle warning signs: Discoloration of the cleaning solution, the lack of a dilution line in a reprocessing sink to indicate the proper dilution of a cleaning agent, fluid invasion that is apparent under the eyepiece of a flexible endoscope, or residue found in the bottom of a flexible endoscope storage container. Not finding any cleaning brushes in the decontamination area to be used for endoscopes with lumens is another indication we notice during site visits. In addition, opening the sterilization container and finding equipment fully assembled that should have been separated for effective cleaning and sterilization is equally troubling. It is also important to ensure that light post adaptors have been removed from the endoscope for sterilization.
We have sometimes had the experience of asking someone to explain why they clean an endoscopic piece of equipment in a certain way, only to have them respond that “so and so” said to do it this way, or it’s the way we’ve always done it. It is very important to keep in mind that every scope from every manufacturer should be considered a distinctive device with IFU that apply only to that product.
Crit Fisher, Director, Field Ops, Protection1, KARL STORZ Endoscopy-America Inc.
- Endoscopes not being reprocessed within the established OEM recommended time frame encourages biofilm to develop that may be difficult to remove from the endoscope channels under the reprocessing guidelines recommended by the IFU from the respective OEM vendor. These delays are sometimes not flagged and associated with the contaminated scope as it travels thru the reprocessing cycle. This window of delay can go unrecognized to the reprocessing technician.
- Contaminated damaged scopes (i.e., endoscopes damaged during OR use, sometimes are not flagged as having a problem) go unnoticed during the reprocessing cycle. If there is a leak failure, special leak failure protocols are sometimes routinely abandoned and the scope may in some instances not be cleaned per the OEM IFUs for many days prior to shipping to a designated repair facility (adding additional days). This delay contributes significantly to the biofilm development within the endoscope, contributing to the inability to sterilize the instrument.
- Endoscopes can have fluid invasions and still pass a manual leak test and an AER leak test.
- OEM air leak testers may create the appearance of working correctly but not producing the required air flow or no air (noise but no air) to perform the leak testing, contributing to an inconclusive (no leak) test when there is a leak. Decontamination leak testing areas can be noisy and distracting while tests are being performed encouraging improper screening.
- Endoscopes bypassing the decontamination area upon returning from the OR that have been exposed to a patient occupied OR room but not used on a patient can be inadvertently used again on another patient without being properly “cleaned.”
Jon Whinnery, CHL, CSPDT, CRCST, CIS, CNA, Operations Manager, Paces MedEquip LLC
- Visual change in water quality
- Expired chemicals
Angela Maxwell, Senior Clinical Operations Consultant, Cardinal Health
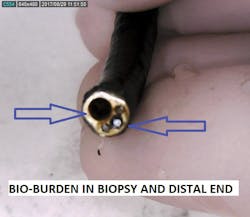
- Bioburden present on/in biopsy channel walls
- Cleaning brush bristles and/or sponge particles present after cleaning
- Use of expired cleaning verification tools that could yield inaccurate test results
- Residual moisture present inside of the endoscope after appropriate drying/hang time has been carried out
- Bioburden present in or on distal optics
- Bioburden present in the bottom of the endoscope cabinet where scopes hang
Brianne Flesher, CFER, Product Manager, Mobile Instrument Service & Repair, Flexible Endoscope Repair Division
- Scopes are not maintained correctly in cabinets, may touch one another, come in contact with cabinet doors, walls and floor
- Caps and valves may remain in place — either the staff are not aware that they must be removed or the soiled caps may have been placed back again post-cleaning
- Scopes remain in cabinets and exceed the recommended staging period (7 days) before reprocessing
- Endoscopes are stored “wet” versus the gold standard “dry”
- Pre-cleaning standards are not adequately followed
- Cleaning brushes are not adequately cleaned or disposed of appropriately
- AER and storage cabinets are not effectively monitored, cleaned and tested for cleanliness
- Inadequate equipment is available for cleaning and requires staff to assume that they must use substandard equipment to clean instrumentation and scopes
- Cleaning audits are randomly completed or follow the standard for audit tools
- Cleaning documentation is not adequate, reviewed or discussed with epidemiology professionals
Melanie Miller, Healthcare Value Management Experts Inc.
The things that need to be addressed here are items such as the adherence to standards, policies and recommended practices. These include:
- Shortcuts taken by cleaning personnel in order to save time
- Proper dilution ratios of enzymatic cleaners not being used
- Temperature parameters not met
- Wrong size or overused brushes being used
- Human error or lack of education. No accountability
- Not following manufacturers IFUs
- Improper or false documentation
- Not verifying manual cleaning practices
It is extremely important for every nurse or technician to understand the importance of proper cleaning and following current practices. Initial education followed by ongoing competencies is crucial as well as auditing of the personnel and processes in place.
Robert B. Dybec, RN, CPSN, CNOR, OperatingRoom Nurse Manager at Winthrop-University Hospital, Mineola, NY,and a clinical consultant, Ruhof Corp.
- Inspection with magnification shows residue still on device
- Inspection with borescope (telescope shows visual residue inside lumen(s)
- Test for protein shows contamination
- When actuating moving parts, they are difficult to move, stick or don’t move at all
- Incorrect brush size does not remove soil inside lumen(s)
Donna Swenson, President and CEO, Sterile Processing Quality Services Inc.
- Film or discoloration on the insertion tube or distal tip
- Chipped, burned or cracked C-covers are places where microorganisms can harbor
- Pitted or missing adhesive around the light guide or objective lens
- Missing adhesive over the distal set screws on the C-cover
Chris Antonucci, Director of Marketing, STERIS Infection Prevention Technologies
- Scope not functioning properly, due to unclear or smeared scope image, leaks or broken locks or tags on scope buckets
- Fluid droplets within the scope lens
- Caps still on scope when taken out of storage
Alicia Titzmann, Senior Clinical Operations Consultant, Cardinal Health
Non-visible blood, mucous, and stool — patient test results may return a false positive due to previous patient’s body debris remaining in the internal workings of the scope.
Jean Sargent, President, Sargent Healthcare Strategies
Read on…

Rick Dana Barlow | Senior Editor
Rick Dana Barlow is Senior Editor for Healthcare Purchasing News, an Endeavor Business Media publication. He can be reached at [email protected].