While sterile processing follows a logical series of steps — decontamination, cleaning and sterilization — so only safe and effective instruments reach the hands of the surgeon, there are a variety of nuances that can jeopardize instrument quality. In this article, HPN highlights best practices in QA sterility assurance and instrument maintenance, as well as new products and services in these areas.
Start with staffing
There is no doubt that the equipment used in the central sterile/sterile processing department (CS/SPD) is critical to ensuring instrument sterility, but the human factor is equally — if not more — important. Scott Zygadlo, Project Director, Hospital Corporation of America, West Florida Division, comments on the need for staff balancing in the CS/SPD, stating:
“SPDs tend to concentrate the bulk of staffing on day shift. This practice doesn’t maximize instrument availability and is misaligned with the product flow to and from the OR. On average, it takes four hours to properly decontaminate, assemble and sterilize sets. If the OR starts at 7 a.m., instrumentation begins returning to decontamination between 10 and 11 a.m. Staff starting at 7 a.m. will be underutilized. The bulk of assembly work begins at 11 a.m. and continues into the afternoon shift. Staffing must be highest between 11 a.m. and midnight for most hospitals. Lack of staff balancing can lead to high rates of immediate use steam sterilization (IUSS). High IUSS rates indicate improper cleaning of instrumentation; a concern for infection control.”
Zygadlo says that staff balancing is relatively simple to perform and can yield big benefits. He says the key is determining the quantity of trays arriving in decontamination each hour. Zygadlo explains that instrument tracking systems generate time/date stamps associated with decontamination for each tray — information the CS/SPD can use to measure the flow of trays. If no instrument tracking system is available, he points out how CS/SPD staff can use a simple tick sheet at decontamination to track the number of trays arriving per hour.
“The key is determining when trays arrive in decontamination, not when they are assembled,” said Zygadlo. “By analyzing this data for a one-week period, you can determine how instruments flow. Reassign your decontamination and assembly staffing to match this flow. The goal of the SPD should be to have zero trays awaiting assembly for first case start.”
Clean is critical
We’ve all heard it time and time again — if it isn’t clean, it isn’t sterile — but do all CS/SPD put this theory into practice? “Sterilized dirt is still dirt — it’s not better or cleaner and it can still cause considerable harm to patients,” says Lindsay Brown, Clinical Educator, Key Surgical, adding: “This is a challenge that many SPD departments face in terms of sterility assurance, quality assurance and instrument maintenance. A SPD tech can do everything they can to follow the instructions for use during the sterilization step of reprocessing, but if an instrument isn’t clean, no IFU-abiding tech can render that instrument sterile. Proper cleaning is the very first critical step in ensuring the success of any sterilization cycle.”
According to Brown, “focusing solely on using the correct sterilization cycle is like putting the cart before the horse.” She notes how there are many important steps that need to be taken prior to sterilization to ensure the success of it. This includes having the correct assortment of tools to ensure instruments are properly prepared for sterilization. She cites ANSI/AAMI ST79:2010, section 7.5.6: “Instruments with lumens should be brushed using a brush that is of the correct size for the lumen … Brushes should be … the appropriate size and bristle type.”
“Proper pre-cleaning processes in the OR and cleaning brushes that are the correct length and diameter will help SPD techs prepare instrumentation for a successful sterilization process and will also ensure instruments are not damaged during manual cleaning,” said Brown.
“Any cannulated instrument is a challenge,” adds Dan DeShane, Regional Product Manger, Surgical Instruments, STERIS Instrument Management Services. “The importance of using the right size brush for the right size hole is extremely important when maintaining cannulated instruments. If you use too small a brush, it won’t adequately scrub the interior. If it is too large, it will fold down and spring open at the opposite end, again inadequately scrubbing the interior. It is very important to have your brushes accessible and labeled so that they can be used effectively.”
Featuring a protective tip and available in lengths up to 24 inches, Key Surgical’s acrylic tip channel cleaning brushes are designed to give the user a flexible handle to maneuver through complicated bends and curves of a surgical instrument. Antimicrobial nylon bristles dislodge debris as the brush is guided through the channel/lumens and an acrylic tip end helps provide extra protection from any potential damage or scratching during the cleaning process. Bristle diameters range from .062 inches (1.58mm) to .44 inches (11.18mm). Bulk packaging is available as well.
Getting down to the details
As medical devices become more complicated in design and also function, the challenge to effectively reprocess them and render them safe and ready to use on the next patient also becomes more complex, points out Ralph J. Basile, Vice President, Healthmark.
“The complexity is along two major dimensions. First is the complexity of the individual device — the difficulty to clean and to be able to inspect for proper cleaning; the complexity to take apart and put back together; the complexity to ensure that the device is functioning properly,” said Basile. “The second dimension of the complexity is a result of having so many complicated, different devices to reprocess. Each one requires its own unique set of steps. Staff needs to be educated and competent; equipment needs to be capable and properly programmed; and in the end the department manager must be able to verify that the process is indeed producing devices that are safe and ready to use.”
Basile notes that with any process, whether it is simple or complex, it is always important to verify that the goal of the process is indeed being achieved. He says this needs to come from monitoring key parameters of the process (e.g., time, temperature, detergent concentration, competency) and also the outcome of the process — namely that devices are clean, sterile, safe and ready for use. According to Basile, the way to achieve both is to implement various verification measures, such as competency testing, temperature monitoring, random and routine inspection/testing of devices, etc.
“Hands down, laparoscopic instruments and kerrison rongeurs pose a major challenge,” added Mike Vargo, Regional Product Manager, Surgical Instruments, STERIS Instrument Management Services. “If the instruments do not have the capability to be taken apart, they pose an extremely high risk of not being completely cleaned, even if the IFUs are followed perfectly. The only way to properly inspect and clean non-take apart laparoscopic instruments and kerrison rongeurs would be to have a trained repair technician disassemble the instruments to gain access to the drive rod (laparoscopic) and inner rail (kerrison).”
Healthmark just introduced MAG310 to its line of enhanced visual inspection products. AAMI ST91 recommends the use of 10x magnification to inspect flexible endoscopes. The MAG310 is a lighted, handheld magnifier that has three lenses: 3x, 10x and 55x, to allow for easy and convenient enhanced visual inspection of many medical devices. It is inexpensive, easily fits in pockets and comes with batteries already installed.
Preventing problems
Another challenge CS/SPD professionals face in quality/sterility assurance of their sterile processing efforts is protecting instruments from damage and detecting device failures before they reach the operating room (OR). As Janice Reed CRCST, Interim SPD Manager for B.E. Smith points out: “Patients’ safety is the No. 1 priority. Do no harm!
“Clean instruments are the first requirement for assuring sterility,” said Reed. “Instruments that are cracked, pitted, rusted and/or stained cannot be cleaned. Hence, sterilization cannot be assured. Malfunctioning instruments due to being worn, loose/missing parts, and being placed in sets are a safety hazard to patients and place the facility at risk for being held liable for patient injury and/or patients acquiring a surgical site infection (SSI).”
According to Reed, scheduled preventative maintenance on a routine basis on all instruments alleviates the majority, if not all of, these problems. “Having a preventative maintenance plan will ensure your patients are safe and ensure your staff and co-workers (OR staff, surgeons and administration) remain pleased with SPD services,” she added.
“Surgical instrumentation represents a large portion of the surgical services budget,” said Zygadlo. “Improper maintenance shortens the life of all types of surgical instrumentation. More importantly, patient safety is jeopardized if a critical instrument fails during a procedure. Although most SPDs utilize a combination of mobile van and depot service to provide instrument care, items typically reach the point of failure before being released for service. Repair is a reactive process that reduces instrument life, increases OR staff and physician dissatisfaction, and creates potential for harm to the patient. Instrument maintenance is a proactive process where items are released for service on a scheduled basis for inspection, repair and refurbishment. Adoption of a true instrument maintenance program minimizes the negatives associated with a reactive approach.”
“The biggest challenge I see in hospitals is not fully utilizing the repair contracts that everyone has,” said Vargo. “As soon as a scissor is dull, a curette flattened or an osteotome mishapen it becomes a patient safety risk.”
“Many facilities still do not look at maintenance from an ‘entire inventory’ standpoint — many times they are just having service on sets that are available and ‘down for reprocessing’ in the department when the service team shows up,” said Deshane. “This is not the best way to use your hard-fought-for budget and leads to surgeon satisfaction issues, patient safety concerns and higher spend on buying new equipment.”
Use your IFUs
CS/SPD staff should inspect every surgical instrument for proper cleanliness, functionality and sterility according to the manufacturer’s instructions for use (IFU) every time they are used. It is a daunting task to achieve in the real world, points out Bob Marrs BA, CRCST, CIS, CHL, Director Consulting Services/Field Operations, Aesculap.
“CSD staff are confronted with multiple challenges ranging from time constraints, equipment backlogs, staffing shortages, overloaded sets to inadequate education/training and a lack of access to IFUs,” said Marrs. “If I had to pick one challenge that impacts CSDs the most it would be adherence to manufacturers’ IFUs. This impacted me personally as a CSD staff member and leader. It also is something that I find in almost every CSD that I visit around the country. It takes a dedicated surgical team and CSD leadership to collaborate through scheduling, communication and teamwork to achieve success with IFUs.”
According to Marrs, overcoming the challenges surrounding manufacturers’ IFUs is not impossible, but takes hard work, communication and understanding from both teams in the perioperative setting (CS/SPD and OR). He notes that a review of IFUs and the instruments/sets with which they are associated can help determine if the facility has the proper amount of equipment in its CS/SPD.
“This information can be collected and presented to your administrators to justify capital purchases,” said Marrs. “Close communication and collaboration with the surgical team will ensure that the right instruments and sets are available when needed. Proper scheduling is extremely important to ensure instrument/set availability. Right sizing your instrument fleet through standardization and optimization is vital to the successful achievement of good quality outcomes.”
Aesculap’s Surgical Asset Management Program has been implemented in hospitals and health systems around the world, helping facilities increase both the clinical and financial value of their surgical instrument inventory and sterile processing practices. The first step in this process is the QuickScan assessment service. This four-day service provides a low-risk, low-investment analysis of the state of instrumentation, reprocessing practices and opportunities for improvement within sterile processing and the OR. The results of this assessment lead to recommendations from Aesculap’s team of SPD consultants on how to further standardize and optimize instrument assets and practices. On average, Aesculap has found that hospitals have far more instruments than are regularly used, resulting in an average instrument reduction of 17 percent after optimization.1
Adequate inventory
When it comes to the quality and sterility assurance involving instrument maintenance, having enough time and sets are a challenge, explains Amanda H. Coss, CSPDS, CSPM, CRCST, CIS, CHL, National Education Coordinator, Mobile Instrument Service & Repair.
“Healthcare is a very fast paced, demanding environment and surgery schedules change almost instantaneously due to its nature,” said Coss. “When instruments are on a maintenance schedule and are limited due to scarcity, the unpredictability of the surgery schedule can be difficult to release the one-of-a kind or limited trauma sets for maintenance.”
Coss says having a routine and up to date instrument management system in place is vital — whether the facility or its instrument repair vendor manages it. For the one-of-a-kind sets, Coss recommends scheduling maintenance based on OR block times and when the specific specialty associated with that set is not operating.
“To keep those limited sets such as trauma on a routine maintenance program, work with your set vendor — such as your trauma set representative to arrange for loaner instrumentation for the just in case emergency, to ensure all your sets can receive routine maintenance,” said Coss. “On-site repair services offer flexibility and help you in these situations as the turnaround time is the same day for most instruments.”
Cleaning validation
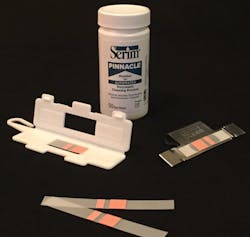
Because dangerous microbes are invisible to the human eye, it is not enough to perform proper cleaning techniques alone. CS/SPD must also take subsequent steps to verify that their efforts were successful. Greg Sautter, Product Sales Manager, Serim Research Corporation, says that understanding the need for appropriate use of cleaning monitors, also known as challenge devices or cleaning verification products, is an important step in assuring proper instrument cleaning and sterilization. He explains how these devices help SPD staff monitor and ensure performance of their washer-disinfectors and ultrasonic cleaners.
“Testing cleaning verification products under a variety of conditions helps SPD staff understand how the current products respond to different factors affecting overall instrument cleaning,” said Sautter.
Serim Research has developed two cleaning verification products designed to monitor enzymatic detergent activity. The PINNACLE Monitor for Automated Enzymatic Cleaning (AEC) is designed for use in both washer-disinfectors and ultrasonic cleaners. This test has been shown to be the most sensitive to changes in the enzymatic activity and concentration of the detergent along with changes in wash cycle time, temperature and mechanical action of the cleaning unit.2
The PINNACLE Monitor for Manual Enzymatic Cleaning (MEC) is the only product available for determining the presence of active enzymes in manual bath solutions using enzymatic detergents. Enzyme activity in manual solutions is dependent upon concentration, temperature and exposure time.
An indication of sterility
Sterilizers are the workhorses of a CS/SPD, processing hundreds, if not thousands, of instruments per day. It is critical to ensure sterilizers are functioning appropriately and effectively killing dangerous microorganisms remaining on devices following their cleaning and disinfection.
Monitoring sterilizers through the use of biological indicators (BI) is a best practice recommended by the Association for Advancement of Medical Instrumentation (AAMI), the Association of peri Operative Registered Nurses (AORN), Canadian Standards Association (CSA) and the Centers for Disease Control and Prevention (CDC). BIs use microorganisms (e.g., Bacillus spores) that are more resistant and greater in number than those microorganisms commonly found on surgical instruments. The BI is placed in a sterilizer load to demonstrate the effectiveness of sterilization. In essence, if the sterilizer can kill the resistant microorganisms in the BI, it is also capable of killing other nasty bugs residing on surgical instruments.
But conventional BIs have their downsides, explains Garry Bassi, Director of MDR for SteriPro Canada. Some biologicals must be incubated for up to 24 hours before the CS/SPD staff can obtain an accurate result.
“With a 24 hour BI test, the hospital cannot use the instruments from that load until the next day,” said Bassi. “Hospitals have to supplement their inventory so that they have the instruments they need on hand, but most don’t have the funding to do this. What I have seen in many hospitals is that staff members commonly bypass the IFU and use the instruments ahead of the 24-hour BI incubation period if the item is needed in an emergency. They have no choice but to do so. This is not good practice and could have fatal impacts in the event of a recall.”
The Advanced Sterilization Products (ASP)” Sterrad Velocity Biological Indicator (BI) System* uses unique and advance optical fluorescence technology to detect biological activity with an incubation time of just 30 minutes. It is the only biological that is fully integrated with all the latest features of Sterrad system sterilizers. Each BI features a unique barcode identifier so when the user scans it to the incubator, the system’s software guides the user as to where to place the BI, and electronically stores the BI’s serial number, lot number and expiry data to prevent usage of expired BIs.
“With the new technology from ASP we are able to save on capital expenditure, we don’t need to supplement and we certainly don’t need to deviate from any standards,” said Bassi. “We can meet standards and continue to turn around our existing inventory within shorter time frames.”
Protecting your assets
Even when CS/SPD professionals take all the necessary steps to clean and sterilize instrumentation, there’s still the risk that instruments will become damaged due to improper handling post-surgery, during transportation, in decontamination and sterilization, and in the storage process.
“The CS/SPD faces the challenge of managing the repair and replacement costs of instruments and analyzing the root cause of recurring cases,” said Marcus Super, Director of InstruSafe Sales and Marketing for Summit Medical. “Identification is only the first step.
Finding a way to solve this issue and reduce unnecessary costs is the largest battle these departments face.”
Super explains that damage can be greatly minimized by understanding the design and benefits of the trays that hold instruments as they are reprocessed, transported and stored. Summit Medical’s InstruSafe Instrument Protection Trays offer both custom and preconfigured tray solutions to organize and protect virtually any instrument set. The trays — made of durable, highly perforated aluminum and silicone instrument holders — secure instruments with 360 degrees of protection during sterilization, transportation, storage and in the OR. These trays have been validated with a variety of sterilization cycles and can be used with both wrap and rigid containers.
Don’t forget data
A key requirement for effective sterility assurance and instrument maintenance is access to accurate inventory records. Andrew Davison, Founder of Nomenclatura stated: “If you cannot identify the manufacturer AND the specific instrument — then you cannot possibly know the detailed manufacturer’s IFUs.
“Global standards, such as ST79 or AS/NZS4187, discuss access to IFU documentation to ensure the validity of sterilization,” continued Davison. “If not followed, the outcome of the sterilizer cycle is mere guesswork, which has no place in the risk adverse healthcare sector.”
Nomenclatura has innovated the traditional approach to instrument inventory data cleansing. The company’s solutions are scalable and it can analyze up to 2,000 instruments per day. Nomenclatura’s fully-scaled data cleansing service is more than 15 times faster than the traditional approach of using in-house clinicians or IT professionals.
“We’re a game changer for SPDs without the high price tag,” said Davison. “To help you assess our service, we can provide a complimentary mini-audit of your instrument inventory. It’s just the start of our tailored approach to ensure we can help you achieve your goals and help you achieve patient safety excellence.”
*Pending 510(k) clearance. Not available for sale or distribution in any market.
References:
- Data on file
- Alfa MJ, Olsen N. Comparison of Washer-disinfector Cleaning Indicators: Impact of Temperature and Cleaning Cycle Parameters. Am J Infect Control 2014;42:e23-e26
Instrument care tips (sidebars):
- Preventative maintenance program tips
- Tips for testing scissors
- Instrument data management tips

Kara Nadeau | Senior Contributing Editor
Kara Nadeau is Sterile Processing Editor for Healthcare Purchasing News.