Flexible Nutrition and Drug Delivery Supports Patient-Centered Care
Chronic conditions, such as diabetes, cancer, cardiovascular, autoimmune, and inflammatory diseases, are on the rise. Biopharmaceutical companies continue to develop new drugs, therapeutics, and nutritional formulations to sustain and save lives.
In parallel, patients are demanding improved delivery modalities that offer ease of use and convenience, particularly for treatments requiring infusion or injection.
Healthcare Purchasing News explores some of the latest innovations in delivery systems for nutrition, drugs, and therapeutics designed to support safe, effective, and compliant patient care.
Nutritional delivery from hospital to home
Adequate nutrition is critical to patient health, whether it is in the prevention of chronic diseases or the management of existing medical conditions.
When a patient cannot consume enough calories and nutrients through the traditional route of eating, enteral nutrition (delivery of calories and nutrients into the gastrointestinal tract) and parenteral nutrition (delivery of calories and nutrients into a vein) are vital interventions.
Advancements in enteral nutrition delivery
Numerous and often complex diseases can lead to the need for home enteral nutrition (HEN), such as swallowing disorders because of neurological diseases, chronic obstructive pulmonary disease (COPD), heart disease, chronic infections, and malabsorption/maldigestion because of liver, pancreas, or intestinal diseases. HEN is typically started during a patient’s hospital stay and continued post-discharge as a long-term home therapy.1
Like any transition of care from a high acuity to a low acuity setting, transitioning enteral nutritional delivery from hospital to the home has its challenges. Rachel Soriano, senior global portfolio manager of Nutritional Delivery/New Product Development at Cardinal Health, spoke to the continued technology innovation in this area, highlighting the Kangaroo OMNI Enteral Feeding Pump:
“As an industry leader in nutritional delivery, the Kangaroo brand has been globally recognized for more than 30 years through its continuous innovation and improved offerings. The Kangaroo OMNI Enteral Feeding Pump was designed to help improve the lifestyle of enteral feeding patients and caregivers from hospital to home through ease of use, versatility, and portability. Kangaroo OMNI is the first and only attitude independent enteral feeding system in the U.S. designed to deliver thick*, homogenized and blended formulas.
“Additional new features include interruption monitoring to display nutrition missed when the pump has been turned off and a night mode that darkens the pump screen in low-light settings to help minimize feeding disruption,” Soriano continued. “By offering new enhancements, functionality, accessories, and options for nutritional delivery, Kangaroo OMNI is also helping enteral feeding patients and caregivers to meet their personalized needs throughout their enteral feeding journey.”
*Thick formula is defined as enteral fluid of smooth consistency that is categorized as level 2, 3, or 4 drinks within the International Dysphagia Diet Standardisation Initiative (IDDSI) framework.
Parenteral nutrition formulations
According to Cecilia Soriano, global president of Infusion Therapies and Technologies, Baxter, parenteral nutrition (PN) is a very dynamic category and a strong focus for the company. She said they are currently seeing shifts in PN formulations to meet the nutritional needs of patients throughout the care continuum.
“One shift is to higher protein formulations,” said Soriano. “With traditional formulations, patients would also be getting additional amounts of fluids, dextrose, and calories along with protein. That could lead to complications, such as hyperglycemia, related to the extra dextrose. To meet patients’ protein targets with less fluid and dextrose, we have brought to the U.S. market our CLINIMIX and CLINIMIX E formulations with higher protein. It contains the highest amount of protein, per liter, in any multi-chamber bag available in the U.S.”
“Another formulation shift we are seeing is with lipids,” Soriano continued. “Historically the formulations available were 100% soybean oil based. We are now seeing more mixed lipid emulsions. The Omega 6 fatty acids in 100% soybean oil formulations could have immunosuppressive effects. To manage that, some clinicians would dose only a few times a week, which could lead to underdosing, underfeeding, and other complications.”
To overcome this challenge, Baxter has developed its Clinolipid 20% lipid injectable emulsion for adult patients. It contains 80% olive oil, which is rich in immune-neutral Omega 9 fatty acids, and 20% soybean oil which provides patients with the needed essential fatty acids. It is the lowest soybean oil and highest olive oil lipid formulation available in the U.S.
Parenteral nutrition delivery
It is not only the formulations that are evolving to meet patient needs, but also the delivery methods, with the transition from individual bags to multi-chamber bags that hold multiple nutrients and components.
Gordon Sacks, PharmD, BCNSP, FASPEN, FCCP, senior director, Medical Affairs for U.S. Parenteral Nutrition at Fresenius Kabi, described the evolution of PN delivery since its development 50+ year ago:
“Delivery systems for PN (parenteral nutrition) have evolved extensively since being invented in the late 1960s. Historically, PN was administered to patients from multiple 1-liter glass bottles and these bottles were typically infused over 8 to12 hours. Dextrose and amino acids were combined in one bottle and lipid injectable emulsions were infused from a separate bottle. In the 1980s, there was a shift from bottles to IV bags for convenience and safety (i.e., avoiding breaking/cracking of glass containers).”
“At the same time, new research established that dextrose, amino acids, and lipid emulsions could be combined into 1 bag container to be administered over a 24-hour period (avoiding the need to infuse multiple containers within a 24-hr period),” Sacks continued. “During this time, PN was exclusively prepared and mixed by a pharmacist in a hospital or by a home infusion company on a daily or weekly basis according to strict aseptic compounding regulations. After preparation, compounded PN bags could be stored up to 9 days under refrigeration or 30 hours at room temperature. After these time periods, the bags would be discarded.”
Sacks highlighted how Fresenius Kabi’s 3-chamber bags (3CB) have simplified the PN ordering and administration process for both clinicians and patients. The bags are designed to deliver three macronutrients (dextrose, protein, and lipids) plus electrolytes in volumes and concentrations that meet the needs of most PN patients.
“Specifically, for clinicians, these products improve the ease and convenience of the prescribing process,”. Sacks explained. “They also minimize misinterpretation of prescription orders and calculations associated with compounded PN preparations. Ultimately, increases in cost savings from decreased pharmacist and prescriber time can result from the use of standardized, commercially available PN products such as Fresenius Kabi’s 3CB PN bags. For patients, the overall administration process is simplified since all nutrients and components are available in one container.”
Infused nutrition on the go
Home parenteral nutrition (HPN) is the primary lifesaving therapy for patients with chronic intestinal failure (e.g., Crohn's disease, short bowel syndrome) and may also be provided to patients as palliative nutrition in the late phases of end-stage diseases (e.g., cancer).2 HPN delivery systems have evolved to support the unique needs of patients receiving this therapy in outpatient settings, including the home.
“We are in the midst of a transformative shift in healthcare with an increasing number of patients receiving care at home,” said Emily Levy, co-founder, president, CBO of Mighty Well, which designs innovative medical products to make life simpler for patients. “Parenteral delivery systems are adapting to meet the unique needs of those relying on parenteral nutrition in a home setting. These advancements go beyond mere convenience; they signify a commitment to personalized and patient-centric healthcare. “
A leading innovation in this evolution is Mighty Well's Fluid Motion Backpack, an infusion backpack that eliminates the need for patients to be tethered to an IV pole for extended periods of time, providing them with greater mobility and dignity in their care.
“This innovation embodies a dedication to combining patient-centric design with compassionate care,” explained Levy. “The Fluid Motion Backpack serves as a powerful symbol of the ongoing revolution in home-based healthcare, reshaping how patients engage with and manage their parenteral nutrition and supplies. It represents the exciting shift towards the empowerment of patients in their continuum of care and is helping to redefine the quality of life for patients relying on home-based parenteral nutrition.”
Nutrition delivery monitoring
Accurate energy needs assessment is important along the patient’s care continuum so that underfeeding and overfeeding do not occur. Energy needs may vary from when a patient is mechanically ventilated, receiving PN in the intensive care unit (ICU) to the point they are discharged and receive PN at home. For this reason, Baxter offers Q-NRG+, a portable metabolic monitor, which provides quick and accurate resting energy expenditure (REE) measurements through indirect calorimetry (IC).
“Indirect calorimetry is considered the gold standard for assessing energy needs,” Angie Davanos, senior director of Medical Affairs, Baxter, explained. “Q-NRG+ can be used to measure the energy needs of the patient throughout their care journey as their conditions and needs change throughout the continuum of care.”
Drug and therapeutic delivery for convenience and compliance
The U.S. Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) approved 55 novel drugs in 2023, as well as approving new dosage forms or drug formulations, and making some prescription drugs available over the counter.3 While some are administered in tablet or capsule form, others require injection or infusion.
New injectable drugs approved in 2023 include Talvey (talquetamab-tgvs) to treat adults with refractory (treatment-resistant) or relapsed (recurring) multiple myeloma who have received other therapies, and Leqembi (lecanemab-irmb) to treat Alzheimer’s disease.
Newly approved infusion drugs include Loqtorzi (toripalimab-tpzi) to treat recurrent or metastatic (spreading) naso-pharyngeal carcinoma (NPC), a rare head and neck cancer, when used together with or following other therapies.
As more treatments expand outside of hospitals and physicians’ offices and into patients’ homes, manufacturers have responded with the development of convenient, easy to use delivery devices.
Home drug infusion management
As noted by the National Home Infusion Association (NHIA), “Infusing medications at home or elsewhere outside of an institutional setting reduces time spent away from normal activities, such as work, school, and hobbies while completing therapy.”4 But as Ben Noonan, general manager, Eitan Medical North America, pointed out, it also has its challenges. He stated:
“Drug delivery can be complicated and tedious. It requires patients to perform several steps to ensure successful administration, such as loading the drug, choosing the right settings and dosage for the specific device, ensuring sterility of both the device and the drug, and recording the dosage amounts and times given. To ensure patient-centric drug delivery in the home, minimizing patient interaction with the drug handling process needs to be a top priority. This step helps prevent basic user errors that can lead to confusion, frustration, and non-compliance.”
“By enhancing ease-of-use factors, such as adding touch screens, intuitive protocol instructions and safety embedded software, home-based patients using either infusion or drug delivery devices will be better positioned for success,” Noonan added.
Last year, Eitan Medical launched Eitan Insights, a cloud-based infusion management system designed to meet the unique needs of home and specialty infusions. Eitan Medical's Sapphire infusion platform, with its universal plug and play cellular accessory Sapphire Connect, and Avoset infusion pump with the AvosetGo app, connect and transmit infusion treatment data to Eitan Insights.
Eitan Insights provides near-real time prescription compliance data and pump geo location, enabling home infusion providers to optimize resources and reduce hospital readmission, helping improve both patient and caregiver experience, according to the company.
Self-injection devices
Application of self-injection devices, including vials and syringes, prefilled syringes, prefilled pens, and auto-injectors, has rapidly expanded to encompass a wide range of conditions and treatments.
In many cases, self-administration of subcutaneous drugs and therapies is more convenient for the patient compared with having to go to a healthcare facility for treatment. Patient administered self-injection is also a more cost-effective solution for healthcare delivery and helps alleviate today’s challenge of clinical staffing shortages.5
For example, biologics are increasingly used to treat specific types of cancer, autoimmune conditions, and inflammatory diseases. While in the past patients had to receive intravenous infusion of a biologic in a doctor’s office or clinic, today technology has evolved so that patients can self-administer the therapy via subcutaneous injection.
Pre-filled handheld autoinjectors are commonly used for self-administration of biologics. These “spring-actuated mechanical devices, activated by pressing a button or pushing against the injection site,” are designed to “deliver a predetermined fixed volume from a prefilled syringe within 10 to 20 seconds.”5
Smart injection devices
Poor medication adherence is a significant problem among patients, with non-adherence estimated at 50% for chronic illnesses. In the U.S., poor adherence is responsible for an estimated 125,000 deaths annually and estimated to cause 10% of all hospitalizations.6
Take Type 2 diabetes, for instance, which affects an estimated 38.4 million people in the U.S. (11.6% of the population) and continues to rise in prevalence.7 Failure to adhere to insulin therapy regimens is one of the greatest barriers to Type 2 diabetes treatment.8
Manufacturers of injectable insulin delivery systems continue to evolve their devices to support better adherence. Delivery mechanisms have transitioned from vial and syringe to prefilled insulin pens, making it easier and less painful for diabetes patients to give themselves injections.
More recently, “smart” or “connected” insulin pens have been developed to help improve therapy adherence. They connect with smartphone applications to calculate and track doses, and provide helpful reminders, alerts, and reports.9 Smart connectivity and digital data collection also offer “insights into the impact of dosing behavior on glycemic outcomes” that can be helpful to diabetes care teams when developing patient counseling strategies.10
Taking it one step further, manufacturers have developed smart pen caps and attachments that can turn disposable insulin pens into smart devices when paired with smartphone applications.
Wearable injectors
Wearable injectors for subcutaneous drug delivery offer convenience for the patient by administering large volumes of drugs or other therapeutics over an extended time. The rising prevalence of chronic diseases such as diabetes, cancer, and autoimmune disorders that require long-term medication management is driving growth in this delivery modality, also known as “on-body delivery systems” (OBDS).11
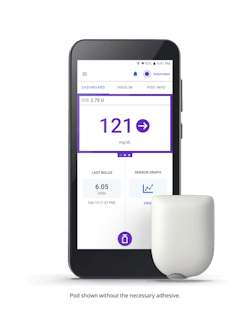
One example is Insulet’s flagship innovation, the Omnipod 5 Automated Insulin Delivery System, which integrates with a continuous glucose monitor to manage blood sugar with no multiple daily injections and zero fingersticks. It can be controlled by a compatible personal smartphone or the Omnipod 5 Controller. The Omnipod Insulin Management System provides a unique alternative to traditional insulin delivery methods. With its simple, wearable design, the tubeless disposable Pod provides up to three days of non-stop insulin delivery, without the need to see or handle a needle.
Researchers report how wearable large-volume injectors open the door to new dosing options for patients. For example, evolocumab, a monoclonal antibody used to lower low-density lipoprotein cholesterol levels can be self-administered once a month within 5 minutes through a patient-loaded wearable large-volume injector, as opposed to every two weeks within 15 seconds using a prefilled handheld autoinjector.12
“Our industry is experiencing a shift of treatment location from hospital to home for chronic diseases, driven by patient demand for more convenient treatment options, and the evolution of on-body delivery systems (OBDS),” said Atul Patel, vice president of Devices and Delivery Systems at West Pharmaceutical Services. “West’s SmartDose On-Body Delivery System* was the first large-volume wearable to be approved as a combination product by the U.S. Food and Drug Administration and is now used with three approved therapies in the market. This technology has since evolved with multiple generations and different sizes with the goal of improving adherence and the patient experience.”
As wearable injector technology evolves, its applications broaden to improve the lives of chronic disease patients. In January 2024, researchers at the UNC School of Medicine announced their creation of a wearable patch featuring electrically triggered microneedles to deliver on-demand treatments for neurodegenerative disorders, including Alzheimer’s disease.13
“The beauty of this device is that it can house dozens, if not hundreds, of concentrated drugs and can program their sequential release automatically,” said lead researcher Juan Song, PhD, professor of pharmacology at the UNC School of Medicine.
Hydrogel injectables
While some chronic conditions can be treated through periodic large dose administration, others require controlled release of drugs over time. The ability to inject a drug and have its effects last weeks or even months is also more convenient for the patient.
Hydrogels have emerged as a modality to facilitate diffusion-based drug release. A drug encapsulated in a biodegradable hydrogel is injected into the patient where the gel slowly breaks down, releasing the drug over a desired period.
Beyond the benefit of controlled release, hydrogel injectables also facilitate more targeted drug delivery to a specific area of the body, for example, injection of immunotherapeutic agents directly into a tumor.
For Type 2 diabetes patients treated with GLP-1 drugs (e.g., Ozempic, Mounjaro, Trulicity, Victoza), a new hydrogel drug delivery system developed by Stanford University “transforms daily or weekly injections” of GLP-1 drugs “to just once every four months.”14
The new polymer-nanoparticle (PNP) hydrogel infused with a GLP-1 drug is injected under the patient’s skin. Over the course of four months, the hydrogel “melts away” releasing the drug molecules into the patient’s body.
Stanford researchers believe “such a system will greatly improve management of both diabetes and weight, improve patient drug compliance, and help those with Type 2 diabetes improve long-term health outcomes.”
References:
1. Bischoff SC, Austin P, Boeykens K, Chourdakis M, Cuerda C, Jonkers-Schuitema C, Lichota M, Nyulasi I, Schneider SM, Stanga Z, Pironi L. ESPEN guideline on home enteral nutrition. Clin Nutr. 2020 Jan;39(1):5-22. doi: 10.1016/j.clnu.2019.04.022. Epub 2019 May 30. PMID: 31255350.
2. Pironi L, Boeykens K, Bozzetti F, Joly F, Klek S, Lal S, Lichota M, Mühlebach S, Van Gossum A, Wanten G, Wheatley C, Bischoff SC. ESPEN practical guideline: Home parenteral nutrition. Clin Nutr. 2023 Mar;42(3):411-430. doi: 10.1016/j.clnu.2022.12.003. Epub 2023 Jan 9. PMID: 36796121.
3. New Drug Therapy Approvals 2023, FDA, January 2024, https://www.fda.gov/media/175253/download?attachment
4. What is home infusion? NHIA, https://nhia.org/about-infusion-therapy/
5. Andreas Schneider, Harald Kolrep, Hanns-Peter Horn, Christoph Jordi, Sina Gierig & Jakob Lange (2023) Understanding patient preferences for handheld autoinjectors versus wearable large-volume injectors, Expert Opinion on Drug Delivery, 20:2, 273-283, DOI: 10.1080/17425247.2022.2162037
6. Baryakova, T.H., Pogostin, B.H., Langer, R. et al. Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems. Nat Rev Drug Discov 22, 387–409 (2023). https://doi.org/10.1038/s41573-023-00670-0
7. National Diabetes Statistics Report, CDC, https://www.cdc.gov/diabetes/data/statistics-report/index.html
8. Skriver LKL, Nielsen MW, Walther S, Nørlev JD, Hangaard S. Factors associated with adherence or nonadherence to insulin therapy among adults with type 2 diabetes mellitus: A scoping review. J Diabetes Complications. 2023 Oct;37(10):108596. doi: 10.1016/j.jdiacomp.2023.108596. Epub 2023 Aug 22. PMID: 37651772.
9. What is a smart insulin pen? American Diabetes Association, https://diabetes.org/about-diabetes/devices-technology/smart-insulin-pen
10. MacLeod J, Im GH, Smith M, Vigersky RA. Shining the Spotlight on Multiple Daily Insulin Therapy: Real-World Evidence of the InPen Smart Insulin Pen. Diabetes Technol Ther. 2024 Jan;26(1):33-39. doi: 10.1089/dia.2023.0365. Epub 2023 Nov 29. PMID: 37855818; PMCID: PMC10794824.
11. Wearable Injectors Market Surges Towards US$ 19.4 Billion by 2033 with 10.2% CAGR | Persistence Market Research, Persistence Market Research, January 22, 2024, https://finance.yahoo.com/news/wearable-injectors-market-surges-towards-090000904.html?fr=sycsrp_catchall
12. Andreas Schneider, Harald Kolrep, Hanns-Peter Horn, Christoph Jordi, Sina Gierig & Jakob Lange (2023)Understanding patient preferences for handheld autoinjectors versus wearable large-volume injectors, Expert Opinion on Drug Delivery, 20:2, 273-283, DOI: 10.1080/17425247.2022.2162037
13. Wireless Drug Patch Shows Promise as Chronic Disease Treatment Delivery System https://news.unchealthcare.org/2024/01/wireless-drug-patch-shows-promise-as-chronic-disease-treatment-delivery-system/
14. New drug delivery system could reduce daily diabetes shots to just three a year, Stanford News, November 21, 2023, https://news.stanford.edu/2023/11/21/drug-delivery-system-reduce-daily-diabetes-shots-just-three-year/
*West’s SmartDose on-body injector platform is not independently cleared or approved by any regulatory body for general healthcare professional or patient use, nor is it available for general commercial purchase. Its distribution and use are subject to applicable regulatory requirements for clinical investigation, and for marketing authorization, as used in combination with a specific drug or biological product. Compatibility with any particular drug or biologic must be confirmed, and its ability to achieve the desired patient benefits must also be confirmed, on a case-by-case basis. Important product and safety information and warnings available at: https://www.westpharma.com/products/self-injection-platforms/smartdose

Kara Nadeau | Senior Contributing Editor
Kara Nadeau is Sterile Processing Editor for Healthcare Purchasing News.