The do’s and don’ts of cleaning with chemicals
Patients expect to leave a healthcare facility healthier than when they came in, but healthcare-associated infections (HAIs) and antibiotic-resistant organisms pose a real danger to patient safety and care. These dangers can come from the items used on patients (e.g., non-invasive diagnostic equipment) or in patients (e.g., surgical instruments and devices), and even those in the patient care environment (e.g., high touch surfaces, pens, cell phones).
While disinfectants and sterilants are critical weapons in a healthcare facility’s arsenal when it comes to fighting HAIs, their effectiveness comes down to a number of factors, including proper product selection, safe shipping and storage, and correct usage in conjunction with safety data sheets (SDS) and instructions for use (IFU).
We reached out to manufacturers to obtain their insights on the top factors healthcare organizations should take into consideration when selecting disinfectants and sterilants, as well as the latest trends and products in this area.
Stick to standards for quality and safety
Healthcare organizations have increasingly adopted best practices from other industries, such as manufacturing, to improve quality, efficiency and safety. One key learning has been that process variation increases the risk for errors, while standardization improves outcomes. As quality management expert W. Edwards Deming is quoted as saying: “Uncontrolled variation is the enemy of quality.”
With regards to disinfectants and sterilants in healthcare, experts agree that standardized processes based on industry guidelines and manufacturer recommendations promote effectiveness and safety.
IFUs
Central sterile/sterile processing department (CS/SPD) professionals and manufacturers acknowledge that the manufacturer’s IFU is the ultimate guide to sterile processing.
As Melinda Benedict, MS, CIC, CFER, Manager of the Infection Control Program for Olympus Corporation of the Americas, points out, the IFU includes recommendations for detergents, disinfectants and sterilants used in the processing of the item.
“It is most important that those responsible for reprocessing instruments follow the FDA-validated reprocessing instructions provided by the manufacturer,” said Benedict. “The integrity and safety of instruments is dependent upon the instructions being followed carefully.”
Safety data sheets and labels
But it’s not enough to follow the instructions for the instruments being processed. Healthcare staff members must also familiarize themselves with the disinfection and sterilization methods being used. That’s where safety data sheets (SDS) and product labels come in.
While healthcare organizations are focused on the safety and care of patients, they can’t forget the safety of their own staff, especially when it comes to disinfectant and sterilant use. According to the World Health Organization (WHO), hospital cleaners and disinfecting chemicals can cause respiratory and reproductive disorders, eye and skin irritation, central nervous system impairment, cancers and other human health effects.1 These products are designed to kill. While that’s beneficial for eradicating dangerous microbes, it presents a real danger to the healthcare staff using them.
Under the U.S. Occupational Safety and Health Administration’s (OSHA) Hazard Communication Standard (HCS) (29 CFR 1910.1200(g)), chemical manufacturers, distributors or importers must provide SDS (formerly known as material safety data sheets or MSDS) to communicate the hazards of hazardous chemical products.2 The SDS includes the properties of each chemical; the physical, health, and environmental health hazards; protective measures; and safety precautions for handling, storing and transporting the chemical.
“Any item that can kill organisms can also harm humans, so consideration must always be taken,” said Emily Lorcheim, Brand Manager for ClorDiSys. “A tool that can better assist a user is to review the SDS, which describes necessary precautions that need to be made during and after application.”
Lorcheim points out how all disinfectants and sterilants used in U.S. healthcare facilities must be registered with the U.S. Environmental Protection Agency (EPA) before sale or distribution; therefore, the product’s EPA label is another good source of information.
“When determining whether or not to choose between a disinfectant or a sterilant, one must first consider how high of a kill level is needed,” said Lorcheim. “Disinfection is defined by the EPA as the kill of bacteria, whereas sterilization is the statistical elimination of all viruses, bacteria and spores, and results in a 99.9999 percent, or 6-log, kill. Sterilization methods are needed for certain circumstances in healthcare such as implantable medical devices, the outbreak of significant disease, etc. An EPA label will provide information such as what it can kill, the level of kill it can provide and usage instructions.”
Staff education
As with IFUs, effective use of SDSs and product labeling information for disinfectants and sterilants requires more than providing staff with these resources and expecting them to follow the instructions. Healthcare organizations must invest in ongoing staff education to promote learning and best practices for process standardization based on these materials.
“Disinfectants and sterilants are registered by the EPA or the U.S. Food and Drug Administration (FDA) and all label instructions are carefully considered and reviewed in order to provide correct usage for the most effective outcome,” said Alison Behn-Gartland, Customer Technical Sales Director for Micro Scientific. “Because these chemicals provide an important task, users should ensure they are reading, understanding and following these labels in their fullness.”
Penny Sabrosky, Clinical Educator for Kem Medical Products, explains how education should go beyond routine disinfectant and sterilant use, to encompass unexpected events such as spills and other emergency situations.
“It is of utmost importance to educate staff to the standard nomenclature of the chemicals being used in applications throughout the department,” said Sabrosky. “In the event of an emergency, staff must be able to correctly identify the chemical being utilized in order to properly reference the SDS. During an emergency, simply referring to brand names of a process, rather than the correct names of the specific chemical substances, can lead to confusion and delayed response times.”
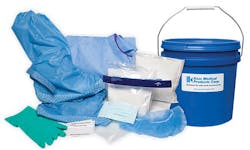
Kem Medical’s KemSure Spill Control Kits provide all components necessary to rapidly respond to small spills of hazardous liquid chemicals used in sterile processing departments. Kits contain Chemsorb Spill Response Pillows to absorb and collect liquid, KemSafe neutralizing powders to deactivate and neutralize hazardous chemicals, and personal protective equipment (PPE) for safe handling and disposal. KemSure Spill Control Kits are for use with glutaraldehyde, OPA, OPA-C and formaldehyde, and are available in packaging choice of pail or tote for storage convenience.
Behn-Gartland adds that disinfectant and sterilant manufacturers are available to help with staff education around their products, stating: “Manufacturers are more than happy to take the time to in-service and answer questions to ensure that chemicals are being correctly used. A mere deviance of a label can result in patient harm; not allowing correct exposure time, not knowing when to test a product or not mixing correctly. It is the responsibility of CS/SPD to guard each patient and protect him or her from exposure to extremely dangerous and growing pathogens that are present in healthcare.”
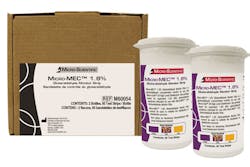
Because all liquid glutaraldehydes/sterilants must be tested before use in order to make sure the chemical is at the proper concentration in order to kill pathogens, Micro Scientific’s glutaraldehyde, Micro-Cide 128 HLD (MC28-04-128), now has its own test strip, Micro-MEC 1.8%(M60054).
Don’t single out solutions
Sabrosky points out that while particular disinfectants and sterilants, such as ethylene oxide, have been singled out for the potential dangers they pose, healthcare organizations must treat all products in this category with care, stating:
“Ethylene oxide is a highly effective method of low-temperature sterilization but it once lost favor as a preferred method of sterilization because of what some considered onerous safety and regulatory issues. In a rush to replace ethylene oxide, various sterilants and high-level disinfectants were marketed as more benign substances that did not require safety and monitoring diligence. However, over the years, as other chemicals have come to market and more data has become available it has become apparent that all sterilants and high-level disinfectants must be utilized with proper policies and procedures in place involving safety, handling, documentation and regulatory compliance. This often includes personal exposure monitoring for OSHA and Joint Commission compliance, as well as emergency action plans pertaining to leaks, spills and evacuation procedures.”
Ship and store safely
Because some disinfectants and sterilants can be heat or cold sensitive, with extreme temperatures impacting their efficacy, healthcare organizations must also educate their staff members on storage and transport. But what is outside of a healthcare facility’s control is what happens to disinfectants and sterilants outside of its four walls, including how they are warehoused and transported to the location for use. That is why solutions for testing product efficacy are important tools, explains Susan Hapak, AB, MBA, President/Owner of Current Technologies.
Test Strips
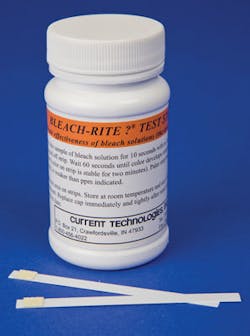
“Bleach-based disinfectants may lose effectiveness if exposed to high temperatures, which is an issue if the product sits on a hot semi-trailer during summer,” said Hapak. “Conversely, hydrogen peroxide disinfectants lose effectiveness in freezing temperatures, so shipping during winter can be problematic. Most disinfectants specify storage at room temperature (55 to 85 degrees), yet non-conditioned warehouses can be outside that range. To ensure safety, CS/SPD can periodically test the efficacy of disinfectants (upon arrival and throughout shelf life) to confirm that shipping/storage has no adverse effects. Our company, Current Technologies, makes an easy-to-use test strip for bleach disinfectants, which determines if the bleach in a bottle or wipe is at the correct strength to kill germs.”
Current Technologies’ Bleach-Rite Test Strips help determine if a bleach solution is at disinfection strength. To check efficacy, the user dips the test strip in the bleach solution, waits 60 seconds and then compares it to a color chart. If the color is dark enough it indicates that the bleach strength is at or above .525 percent (or 5250 ppm, the 1:10 dilution recommended by CDC and OSHA). If the color is not dark enough the bleach solution is not strong enough to disinfect and should be discarded. Bleach-Rite Test Strips can test the strength of numerous brands of bleach disinfectants in varied packaging, including spray bottles and canisters of wipes. They are patented and made in U.S. by a certified, woman-owned small business.
Additional considerations
While there are general guidelines and regulations around disinfectant and sterilant use in healthcare facilities, many complex and high-risk devices require additional consideration and processes to ensure microbes are eradicated and patients remain safe.
Ultrasound probes
Studies have shown how internal probes carry significant risk for cross-contamination between patients even when they are used with disposable covers.3 Even with low-level disinfection performed between use, probes can carry infectious organisms, as evidenced by a study of vaginal ultrasound probes, which found “a considerable number” of endovaginal ultrasound probes contaminated with Human Papillomavirus (HR-HPV) after low-level disinfection.4 Global guidelines now recommend high-level disinfection be performed on ultrasound probes between patient use.5
Ken Shaw, President of North America for Nanosonics, offers the following advice when selecting an infection prevention solution for ultrasound probes:
“It’s important to consider the effectiveness of the technology and its ability to reduce risks of cross infection, as well as safety,” said Shaw. “Equally as important is its environmental impact. Additionally, a system that can be used at the point of care, versus limited to the CS/SPD, is an important consideration. Having a high level disinfection (HLD) device for ultrasound probe decontamination at the bedside significantly maximizes workflow and infection prevention standard operating procedures (SOP). It’s critical that it helps ensure compliance with the latest guidelines and is simple to use, thus encouraging consistent best practices. Automatic traceability for digital disinfection records and IT integration is important, and a system that prevents exposure to hazardous chemicals is essential.”
Nanosonics recently announced trophon2 HLD for ultrasound probe decontamination in North America. The device offers medical professionals a smart solution that helps ensure compliance with the latest guidelines for reprocessing of surface and endocavity ultrasound probes without the use of hazardous chemicals. The system is equipped with an automatic traceability solution that creates audit-ready digital records of each HLD cycle with the option for hospitals to integrate trophon2 into IT systems. As a result, disinfection records are centrally stored and accessed by the entire IT system with the ability to link information directly to a patient’s electronic medical record (EMR).
Beyond instruments and devices
When taking into account the dangers of HAIs and cross-contamination between patients, the risks go far beyond the medical devices and instruments processed by the CS/SPD. Studies have shown that germs are everywhere in the patient care environment – from blood pressure cuffs to cell phones. A recent study in the American Journal of Infection Control found 80 percent of stethoscopes were contaminated by high concentrations of bacteria, while another study found bacterial growth in 77 percent of cell phones and 84 percent of pen samples in pediatric and neonatal intensive care units.7
The ClorDiSys Flashbox is a high capacity tabletop chamber disinfection system that has the ability to fit laptops, keyboards, cell phones, pens, tablets, stethoscopes, blood pressure cuffs and more all at once. It employs ultraviolet light disinfection utilizing the UV-C spectrum of light to kill bacteria, viruses and spores. This manner of disinfection is rapid, chemical free and highly effective. Dosage times are very short and to achieve a 99.99 percent kill of most organisms only requires 30 seconds of UV-C light within the Flashbox.
“In a fast-paced healthcare setting, fast-acting disinfectants with broad-spectrum efficacy, short contact time and positive user experience (e.g., ease of use, available in multiple formats, acceptable odor, good cleaning properties) help drive efficiency and compliance while providing clinical value,” said Allison Buldo, Product Manager, Environment of Care for PDI. “In the fight against infections, material compatibility is critically important. Harsh disinfectants may cause equipment damage, unnecessary cost and contribute to poor clinical outcomes. The chosen disinfectant should have proven compatibility with common healthcare surfaces and devices.”
New Sani-Cloth Prime Germicidal Disposable wipes are powered by PDI’s proprietary ACCELOQUAT formulation, a next generation blend of quaternary ammonium, isopropyl alcohol (IPA) and ethanol. This unique combination delivers speed—a true 1-minute bactericidal, fungicidal, virucidal and tuberculocidal disinfectant—and the power to destroy over 50 microorganisms, including 17 multi-drug resistant organisms (MDRO), such as MRSA, CRE and VRE. Sani-Prime Germicidal RTU Spray has the same rapid broad-spectrum efficacy against top HAI-causing pathogens.
References
- Chemical, biological and radiological exposures, World Health Organization (WHO) http://www.who.int/sustainable-development/health-sector/health-risks/chemical-biological-radiological/en/
- Hazard Communication Safety Data Sheets, Occupational Safety and Health Administration (OSHA), https://www.osha.gov/Publications/HazComm_QuickCard_SafetyData.html
- Kac G, Podglajen I, Si-Mohamed A, Rodi A, Grataloup C, Meyer G. Evaluation of Ultraviolet C for Disinfection of Endocavitary Ultrasound Transducers Persistently Contaminated Despite Probe Covers. Paris, France: Hygiène Hospitalière; 2010 https://www.cambridge.org/core/journals/infection-control-and-hospital-epidemiology/article/evaluation-of-ultraviolet-c-for-disinfection-of-endocavitary-ultrasound-transducers-persistently-contaminated-despite-probe-covers/7299E18F2E283E3FB6136AC3EC67F4B7
- Jean-sebastien Casalegno , Karine Le Bail Carval, Daniel EibachMarie-Laure Valdeyron, Gery Lamblin, Hervé Jacquemoud, Georges Mellier, Bruno Lina, Pascal Gaucherand, Patrice Mathevet, and Yahia Mekki, High Risk HPV Contamination of Endocavity Vaginal Ultrasound Probes: An Underestimated Route of Nosocomial Infection? PLoS One. 2012; 7(10): e48137, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3480505/
- Guidelines for Cleaning and Preparing External- and Internal-Use Ultrasound Probes Between Patients, Safe Handling, and Use of Ultrasound Coupling Gel, American Institute of Ultrasound in Medicine (AIUM), March 25, 2018, https://www.aium.org/officialStatements/57
- New study reveals 80% of stethoscopes are contaminated with infectious bacteria, https://www.news-medical.net/news/20170511/New-study-reveals-8025-of-stethoscopes-are-contaminated-with-infectious-bacteria.aspx
- Saeedeh Haghbin, Bahman Pourabbas, Zahra Serati, and Abdolvahab Alborzi,
- Bacterial Contamination of Mobile Phones and Pens in Pediatric and Neonatal Intensive Care Unit, International Journal of Current Microbiology and Applied Sciences, ISSN: 2319-7706 Volume 4 Number 2 (2015) pp. 75-81, https://www.ijcmas.com/vol-4-2/Saeedeh%20Haghbin,%20et%20al.pdf
About the Author
Kara Nadeau
Senior Contributing Editor
Kara Nadeau is Sterile Processing Editor for Healthcare Purchasing News.