Working together to knock out infection in the healthcare arena
As the COVID-19 pandemic continues to hit communities, healthcare workers continue to show up, suit up and treat scores of infected patients. At the same time, they must also care for the medical needs of many other patients.
The entire hospital workforce – from clinical, environmental services (EVS), central service (CS), infection prevention (IP) and other personnel – put the safety of their patients above all. This means taking several precautionary measures throughout care, including donning and doffing personal protective equipment (PPE), handwashing, disinfection, cleaning and decontaminating equipment and rooms and tracking and preventing infections.
University of Miami Health System reports, “Across the U.S., hospital emergency rooms and urgent care facilities are following enhanced protocols for managing cases of COVID-19 while protecting other patients and healthcare workers. These efforts include disinfecting surfaces, ventilating airspaces, and wearing effective protective gear. At UHealth, staff wear masks, gowns, gloves, and face shields. Masks are supplied to incoming patients. ER nurses are assigned to certain zones. Those caring for COVID-19 patients do not also handle non-COVID-19 patients.”1
Healthcare supply, disinfection, sterilization, device and technology manufacturers share with Healthcare Purchasing News what hospitals and facilities are using and doing for staff hygiene, patient care, device and room cleaning and monitoring and preventing COVID-19 and healthcare-associated infections (HAIs).
PPE support
Masks, respirators, gowns and other medical supplies come in high demand as COVID-19 furthers its course. Hospitals should plan and communicate appropriately for the needs of staff and care, addresses the American Thoracic Society. “Hospitals facing a growing population of COVID-19 cases need a coordinated approach with a multidisciplinary team to increase efficiency, conserve PPE and protect staff.”2
PPE, cleaning and other protective interventions made on a facility-wide level support the fight against COVID-19 and other infections, observes Caitlin Stowe, MPH, CPH, CIC, CPHQ, VA-BC, Clinical Affairs Research Manager, PDI Healthcare.
“Since the beginning of the COVID-19 pandemic, we’ve seen healthcare workers come together like never before to protect themselves, patients and visitors. This extraordinary teamwork is occurring through implementing enhanced cleaning protocols, adhering to strict hand hygiene, ensuring appropriate use of PPE and safely distancing patients and visitors, while prioritizing patient care. All of these efforts are ensuring that the risk of contamination of surfaces with SARS-CoV-2 and pathogens is greatly decreased,” Stowe said.
Ansell Healthcare Global Business Unit is ramping up PPE production to meet the demand for frontline workers, informs Gina Gilbert, BSN, RN, Senior Director, Professional Education & Clinical Affairs.
“Because our products are critical to protecting the lives of healthcare providers, emergency responders and other essential workers, we are working non-stop to expand our manufacturing and distribution capacities to ensure essential PPE gets where it needs to go. Ansell is also focused on providing education on COVID-19 recommendations and the proper use of PPE products (https://www.ansell.com/us/en/the-new-coronavirus). The new guidelines for PPE protection with COVID-19 will likely become standard practice for the foreseeable future. Healthcare facilities will focus on maintaining necessary reserves of essential equipment and the demand for disposable products will continue. Protection may also become more commonplace for the general population,” Gilbert explained.
STERIS provides solutions for decontamination and sterilization to help achieve safe environments for care.
“Mitigating risk is a primary responsibility for all healthcare professionals,” said Tamara Behm, MSN, RN, CIC, Clinical Education Specialist, STERIS Infection Prevention Technologies division. “Following CDC and OSHA guidelines regarding proper hand hygiene and wearing facemasks provides a strong foundation. Beyond that, we’ve been speaking to customers about: decontaminating compatible N95 or N95-equivalent respirators, requiring facemasks for those with respiratory symptoms and considering expanding hours of operation to limit crowding during peak hours. In addition, we are recommending customers look at their processes and re-evaluate workflow in reprocessing spaces to ensure there is no risk of cross contamination.”
The widespread shortages of PPE during COVID-19 prompted the U.S. Food and Drug Administration (FDA) to work with relevant sterilization/decontamination companies and grant emergency use authorization (EUA) to decontaminate N95 or N95-equivalent respirators in the U.S. for reuse by healthcare workers in hospital settings.3
Dominic Ivankovich, President of ASP, declared, “On 4/11/20, ASP received EUA from the FDA for the use of STERRAD Sterilization Systems to decontaminate compatible N95 respirators. Our STERRAD systems are already available onsite in many U.S. hospitals and could collectively decontaminate millions of masks. ASP is confident this newly available option will help mitigate PPE shortages in the event of future outbreaks.”
Hand hygiene and infection monitoring
Healthcare and hospital staff keep a constant pulse on hand hygiene, cleaning, sterilization and protection against patient infections. Martin McGonagle, General Manager, Healthcare, SC Johnson Professional, calls out routine handwashing, hand disinfection and hand hygiene compliance.
“One of the best ways to prevent the spread of germs is frequent handwashing with soap and water. In addition, healthcare institutions are ensuring hand sanitizer is readily available and educating users to help protect staff and patients. The Alcare Extra Foaming Hand Sanitizer uniquely contains 80% Ethanol, which is now recommended by the WHO.4 This ABHR product can eliminate up to 99.9999% of tested organisms in 15 seconds – information about the specific microorganisms is available on file,” McGonagle stated.
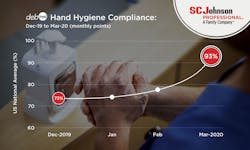
“Since the arrival of COVID-19, we’ve seen a steady increase in hand hygiene compliance rates in many hospital units utilizing the DebMed Hand Hygiene Monitoring System, according to the WHO 5-Moments. To reduce the risk of comorbidities, healthcare facilities will implement improved methods for monitoring staff hand hygiene compliance. A greater emphasis will be placed on hand sanitizer formulations that include 80% Ethanol and set a higher standard for reducing HAIs and preventing HAC penalties,” he added.
Digital systems, like the HealthConnex cloud-based infection control software used in skilled nursing and assisted living facilities, help track and report infection cases in real time, said Alex Hunter, Senior Manager, HealthConnex Inc.
Juan-Carlo Cruz, an infection control practitioner, appreciates the HealthConnex product. “The software helped us improve our infection control practices resulting in significantly reduced outbreaks. The app was able to present a clear picture of infection cases on my units. I really like being able to view live updates on floor plans and run multiple reports in graph and tabular formats in seconds.”
Helping to monitor HAIs in facilities and increase efficiency in care is the BD MedMined Surveillance Advisor, explains Clint Pridgen, Platform Leader, MedMined for BD.
“BD MedMined Surveillance Advisor, part of the HealthSight analytic suite, combines ongoing clinical surveillance of HAIs with clinical support and educational tools. This solution helps hospitals optimize their workflows, streamline regulatory reporting and enhance day-to-day infection prevention. As a result, clinicians are empowered to spend less time on administrative tasks and more time on patient care,” Pridgen said.
Fighting surgical site and healthcare-acquired infections
Healthcare teams take many steps to provide safe care for patients and block pathways for surgical site infections (SSIs) or healthcare-acquired infections (HAIs). Not only do these infections strike a dangerous blow with patients’ health, they also increase costs of care, length of stays and chances for deaths.
The WHO looks at curbing these infections as a priority around the world, pointing out its “Global Guidelines for the Prevention of Surgical Site Infection” in a news release.
“Surgical site infections are caused by bacteria that get in through incisions made during surgery. They threaten the lives of millions of patients each year and contribute to the spread of antibiotic resistance. In low- and middle-income countries, 11% of patients who undergo surgery are infected in the process. But surgical site infections are not just a problem for poor countries. In the United States, they contribute to patients spending more than 400,000 extra days in hospitals at a cost of an additional US $900 million per year.”5
The release continued, “The guidelines include 13 recommendations for the period before surgery, and 16 for preventing infections during and after surgery. They range from simple precautions such as ensuring that patients bathe or shower before surgery and the best way for surgical teams to clean their hands, to guidance on when to use antibiotics to prevent infections, what disinfectants to use before incision, and which sutures to use.”
One Syracuse, NY-based healthcare system developed new processes and education in care to reduce their numbers of HAIs, reported the Association for Professionals in Infection Control and Epidemiology (APIC).
“Looking to decrease healthcare-associated infection (HAI) rates across their healthcare system, infection control practitioners in Syracuse, NY identified chlorhexidine gluconate (CHG) bathing as a means of reducing infection rates. After conducting thorough staff training, hospital-wide use of CHG bathing for every patient was implemented, leading to significant results: a 65 percent reduction in central line-associated bloodstream infection (CLABSI), a 30 percent reduction in catheter-associated urinary tract infection (CAUTI), a 100 percent reduction in Methicillin Resistant Staphylococcus Aureus bacteremia (MRSA), and a 28 percent reduction in Clostridioides difficile (C. diff). Estimated total cost savings fell just shy of $515,000 between April 2017 and March 2018.”6
The report added, “In addition to bathing the patients, staff implemented Agency for Healthcare Research and Quality (AHRQ) recommendations to clean patient devices with CHG during the process, including cleaning external catheters six inches down from the patient, as well as lumens of central lines. Educating staff about the CHG bathing was key to ensuring compliance. To alleviate concerns about potential skin side effects using this bathing method, the team incorporated a skincare bundle, including lotions, in the process.”
What are the latest products and practices used in surgical care and infection prevention in hospitals and other healthcare settings? Here are a few examples:
Ventilator and respiratory care
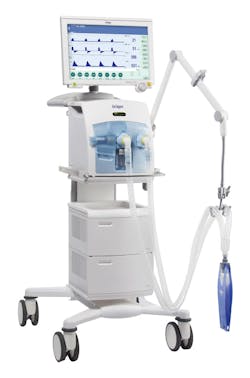
Dräger’s portfolio of ventilators provides respiratory monitoring and vital breathing support for patients in care.
With the ongoing spread of COVID-19, Dräger makes several recommendations on the reprocessing and disinfection of anesthesia equipment to help keep patients safe from infection in an online letter to customers.
“The novel coronavirus (SARS-CoV-2) belongs to the category of enveloped viruses that in principle can be removed with disinfectants with limited virucidal effectiveness. However, for a higher safety level it is also possible to use locally registered hospital disinfectant with a label claim for a non-enveloped virus (e.g. norovirus, rotavirus, adenovirus, and poliovirus). Reprocessing of products, components and surfaces potentially contaminated can be achieved by following the standard procedures described in the Instruction for Use (IFU) and the usage of suitable disinfectants with at least limited viricidal effectiveness.”8
Additionally, Dräger is ramping up production of its masks and ventilators to help meet the rising needs of protection for healthcare workers and care for patients, states the company’s online frequently asked questions.
“There has been a significant increase in global demand for personal protective equipment, especially FFP masks, half masks, particle filters, safety glasses and protective suits. We produce our masks in Sweden and South Africa. Our production capacities are currently fully utilized and we naturally have the relevant back-up stocks to cushion short-term fluctuations. In our Medical Technology division we are currently producing almost twice as many ventilators as before. We are working flat out to expand our production capacity even further. In times of pandemic, we are doing everything in our power to meet our social responsibility to provide for society – worldwide.”9
Wound care
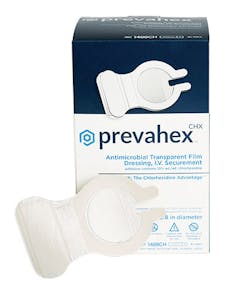
The emergence of drug-resistant microorganisms and recent outbreaks like COVID-19 are bringing a sharper focus on infection prevention, notes Melanie Waddell, Vice President Marketing, Entrotech Life Sciences, Inc. The company’s PrevahexCHX Antimicrobial Dressing helps protect wound and catheter sites for up to seven days and helps ward off infections.
Catheter care
Last year, Dale Medical Products, Inc. (Dale) added its new Hold-n-Place Catheter Securement Devices. They are available as stand alone Engineered Stabilization Devices (ESD) or packaged with transparent dressings or with Entrotech Life Science’s PrevahexCHX(tm) Antimicrobial Transparent Film Dressing to protect patients from infection, according to a release from Dale.
“Like the Dale Hold-n-Place General Purpose Securement Devices, the new catheter securement products are Engineered Stabilization Devices (ESD) and feature a soft, comfortable, flexible design with no hard plastic parts. No skin prep is required for application, and no alcohol is required for removal. The devices are available in two sizes: one for IV, arterial and mid-line catheter securement and another for CVC, PICC and arterial sheath securement. Holdn-Place is the first and only catheter ESD available with the PrevahexCHX dressing. Together, the two products combine the effectiveness of an ESD with the first and only chlorhexidine dressing cleared by the FDA with complete antimicrobial protection throughout the entire transparent areas, and with the adhesive strength and transparency clinicians are looking for in a seven-day securement solution.”10
Disinfection and protection
Reusable, reprocessed instruments, like the HEINE USA, Inc. EasyClean Handle and Classic+ Blades, are designed to help defend against infections in surgical care, addresses Christian Berling, HEINE USA, Inc.
Nanosonics’ trophon EPR and trophon2 provide automated high-level disinfection (HLD) for ultrasound probes to help protect patients and healthcare workers from the risk of cross-contamination, highlights Ken Shaw, President of Americas.
“Upscaling infection prevention practices is the new normal, and this applies to ultrasound too. Ultrasound is a frontline triage and monitoring tool during the COVID-19 pandemic, as it provides actionable information rapidly at the bedside. Lung ultrasound is common, and in severe cases ultrasound guided thoracentesis may be needed to help patients breathe. Critical care and maternal fetal medicine settings are acutely aware of the need to reprocess their ultrasound probes following the Spaulding classification. Some facilities have opted for automated HLD for all probes during the pandemic, for an extra margin of safety in these high-risk settings,” Shaw expressed.
PDI Healthcare’s Sani-Cloth disinfectant wipes, Profend swabsticks for nasal decolonization and Prevantics line for skin or needleless access device antisepsis, all aid with infection prevention, a topic that should continue to be addressed among healthcare staff, suggests Stowe.
“I think the future of infection prevention will go back to basics. Educating and ensuring that staff are using the appropriate disinfectant, at the appropriate times to clean and disinfect, on the appropriate surfaces, and observing the correct contact time will be key to reducing surface contamination, protecting staff and patients, and preventing infections even after this pandemic is over. This will require increased funding and attention to infection prevention efforts to achieve continued success,” she stated.
In addition to PPE, Ansell produces patient care, environmental cleaning and sterile processing supplies and equipment that focus on protection of disease transmission, eliminating cross contamination, and healthcare worker and patient safety and protection, points out Gilbert.
“Ansell manufactures an extensive portfolio of surgical, medical exam and specialty gloves for dental, first responders, food safety, environmental cleaning and sterile processing. We offer lab-safety solutions for chemical, biological and physical hazards, including cleanroom applications. We also offer disposable antimicrobial linens for operating room turnover kits, including disposable mops and straps, and other safety solutions such as positioners for pressure injury prevention, patient transfer and repositioning sheets, and a variety of sharps prevention.”
Surface cleaning
“The hidden heroes right now are the EVS workers and compounding pharmacists who are maintaining the cleanest environments possible during the pandemic. In patient care areas, we see increased disinfectant use. In sterile compounding, IPA and PPE top the list. More confidence in cleaning is the emerging priority. More pre-saturated solutions delivering a metered “dose” through mops and/or wipes is one example. Disinfecting chemistries which clean, disinfect and decontaminate surfaces quickly and without damaging what they’re applied to are also in heightened demand,” Schiering indicated.
Doe Kley, Senior Infection Preventionist, Clorox Healthcare, believes all staff should be responsible for maintaining clean and safe healthcare environments. She points to the new Clorox Total 360 electrostatic sprayer with Clorox Healthcare Spore11 Defense Cleaner Disinfectant, which are EPA-approved to kill the top HAI-causing pathogens on hard, nonporous surfaces.
As fingers touch and contaminate keyboards, Deanne VanKirk, National Sales Manager, Key Source International (KSI), stresses the importance of KSI’s disinfectant-enabled LinkSmart keyboard and San-a-Key software.
“Maintaining clean keyboards at the healthcare desktop on a 24/7 basis will be more important than ever, now that we know how easily COVID-19 is spread and its impact on patients. Whereas traditional keyboards are breeding grounds for germs and viruses, our LinkSmart keyboard features an integrated cleaning button that enables frontline healthcare workers to temporarily disable keys for proper disinfection. Our companion software, San-a-Key, provides an onscreen, animated cleaning guide, scheduled cleaning, desktop push reminders and analytics that empower administrators to know the who, when and where of keyboard cleaning. Our keyboards feature a smooth, crevice-free silicone surface that prevents collection of dirt and germs,” VanKirk noted.
While shoes touch and contaminate floors, Maria P. Garces, Marketing Manager, PathO3Gen Solutions presents its Footwear Sanitizing Station that connects to a standard outlet, requires no additional staff and provides a visible sign of 24/7 continuous protection. A handheld wand using the company’s patented Ozone + UVC technology for sanitizing surfaces, objects and more is also in the pipeline.
She reported that after six months of implementing the FSS, AdventHealth Connerton, an acute care facility in Florida, saw a 34% reduction in HAIs. Jeffrey Miley, Pharm. D., CPh., Director of Pharmacy Services, AdventHealth, described, “To help improve our compliance with USP 797 and minimize the risk of pathogens contaminating our clean room, we added to our department’s action plan the PathO3Gen Solutions Footwear Sanitizing Station. The stations are now part of our process that each employee uses prior to entering our clean room. We will continue to utilize the PathO3Gen Solutions Footwear Sanitizing Station in our ante room because the more tools we have to minimize risk for our patients helps us provide safer patient care. Our last air and surface samples were negative for any growth in both rooms.”
No-touch room disinfection
COVID-19 and other pathogens persist and operating rooms, emergency rooms and other hospital rooms need to be turned over and cleaned quickly and thoroughly. Sarah Simmons, DrPH CIC FAPIC, Senior Director of Science, Xenex, calls out its pulsed xenon UV disinfection robots, which can deactivate pathogens and work in five-minute cycles, without damaging materials or equipment.
“Little has changed in the past 20 years in how we clean and disinfect hospitals. Studies show that less than half of surfaces in a hospital room are disinfected when it’s being cleaned and prepared for the next patient, which poses a threat to the next patient or healthcare worker in that room. The coronavirus pandemic is making it evident that more is needed to stop the spread of disease in healthcare facilities. As a result of the pandemic, we’ve seen increased interest from other healthcare facilities, such as urgent care centers, treatment facilities and medical office buildings. When you’re able to disinfect dozens of rooms per day (like you can with a LightStrike robot), it brings the cost down to about $5/room,” Simmons stated.
She continued, “We’ve seen hospitals move their LightStrike robots from the OR to the emergency department so they can immediately disinfect rooms and areas where coronavirus patients are seen/treated. The LightStrike robot was recently proven to deactivate SARS-CoV-2 in two minutes. Our robots are able to quickly disinfect high-touch surfaces where pathogens can linger (bed rails, tray tables, nurse call buttons, grab bars, wheelchairs, etc.) that may be missed during the manual cleaning process. The Mayo Clinic published a study documenting its 47% reduction in C. diff infection rates after it began using LightStrike robots to disinfect rooms on targeted units. Other hospitals, like Baptist Health in Jacksonville and United Hospital Center, began using their LightStrike robots to decontaminate N95 respirator masks. 3M determined that our robot’s intense pulsed xenon UV light would not damage the fit or filtration of N95 respirators,” Simmons explained.
“During outbreaks or pandemics, it is critical to strictly adhere to evidence-based practices for thorough disinfection and infection prevention. This includes manual cleaning with the addition of “no-touch” disinfection whenever possible. “No-touch” disinfection with Tru-D can also be used prior to manual cleaning to provide a cleaner environment for environmental services staff. Studies have shown that up to 50% of surfaces in healthcare settings are not properly disinfected by manual cleaning alone, which increases the risk of infection for anyone entering the room. By adding Tru-D to standard cleaning protocols, all surfaces in a room are disinfected, and the risk to the next patient and healthcare worker is decreased,” Brewer expressed.
As SARS-CoV-2, the virus that causes COVID-19, lingers on surfaces and is easily spread, cleaning and disinfecting entire rooms is critical, stressed David St. Clair, Chairman and CFO, Halosil International. Disinfection solutions such as Halosil’s Halo Disinfection System, which pairs dry-fog delivery with HaloMist (EPA Reg. No. 84526-6) disinfectant, are imperative to help decrease the possibility of infections.
Infection Prevention Product Listings
IP Product Spotlights
References:
1. It’s Still Safe to Visit the ER, Dana Kantrowitz, UMiami Health News, https://news.umiamihealth.org/en/its-still-safe-to-visit-the-er/?_ga=2.253318669.525710327.1588084972-1115072564.1588084972
2. In Preparing for COVID-19 Cases, Plan Early, Communicate Often Says Critical Care Specialists, https://www.thoracic.org/about/newsroom/press-releases/journal/2020/in-preparing-for-covid-19-cases-plan-early-communicate-often-says-critical-care-specialists.php
3. Emergency Use Authorizations, Personal Protective Equipment EUAs, https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations?utm_campaign=2020-04-10%20New%20EUA%20to%20Decontaminate%20Respirators&utm_medium=email&utm_source=Eloqua#covid19ppe
4. Kratzel A, Todt D, V’kovski P, Steiner S, Gultrom M, Thao TTN, et al. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg Infect Dis. 2020 Jul [date cited]. https://doi.org/10.3201/eid2607.200915
5. WHO recommends 29 ways to stop surgical infections and avoid superbugs, Nov. 3, 2016, https://www.who.int/en/news-room/detail/03-11-2016-who-recommends-29-ways-to-stop-surgical-infections-and-avoid-superbugs
6. #APIC2019 Every penny counts: Reducing infections improves care, cuts costs, June 12, 2019, https://apic.org/news/apic2019-every-penny-counts-reducing-infections-improves-care-cuts-costs/
7. https://www.draeger.com/en-us_us/Hospital/Portfolio/Ventilation-Respiratory-Monitoring
8. SARS-CoV-2 and handling of Dräger Anesthesia Workstations, Updated March 24th, 2020, https://www.draeger.com/Library/Content/SARS-CoV-2-and-handling-of-Draeger-Anesthesia-Workstations.pdf
9. FAQ: General Questions, How is Dräger meeting the high demand for protective equipment and ventilators?, Last update: March 27, 2020, https://www.draeger.com/en-us_us/Home/novel-coronavirus-outbreak#news
10. Dale Introduces New Hold-n-Place Catheter Securement Products, May 17, 2019, https://www.dalemed.com/about/news/news-item/dale-introduces-new-hold-n-place-catheter-securement-products/
11. Clostridium difficile spores only
12. Donskey, C., Evaluation of a novel sporicidal spray disinfectant for decontamination of surfaces in healthcare. CloroxPro. Accessed April 3, 2020.
About the Author

Ebony Smith
Ebony Smith was previously Managing Editor for Healthcare Purchasing News.