When looking for ways to reduce healthcare-associated infections (HAIs) healthcare providers must make infection prevention efforts a top priority every day. In some areas there’s still plenty of room for improvement and in others it appears healthcare providers are taking the diligent steps necessary to fight against infection-causing microbes that are proliferating and getting tougher to beat.
Antibiotic stewardship programs (ASPs), for example, are absolutely essential to combating drug-resistant related hospital infections and the list of healthcare facilities that have implemented them is growing every year. Over 94 percent of those working in the infection prevention field who took Healthcare Purchasing News’ annual 2018 Infection Prevention Survey said they have an established ASP. It’s also the No.1 area that respondents said they would like to receive more information about (see Data, diagnosis, drugs, and dedication for HPN’s latest report on antibiotic stewardship programs).
As a result, effective tools and guidance are readily available to help facilities achieve their infection-reduction goals. HAIs may be a serious problem, but they can be tackled with thoughtful attention from every department and a shared willingness to discover and consistently address all potential sources of contamination with evidence-based practices and product solutions. (See Smart equipment choices can help lower HAI incidents for guidance in HAI product purchasing.) “HAI reduction is the result of proper disinfection protocol coupled with a bundle of other interventions including better manual cleaning practices, better antibiotic stewardship programs and better hand-washing compliance,” said Sam Trapani, Founder, CEO, Steriliz, LLC, a UVC room disinfectant manufacturer. “It requires buy-in from EVS, nursing, c-suite, infection prevention and epidemiology staff. And it’s a lot of work.”
The light debate
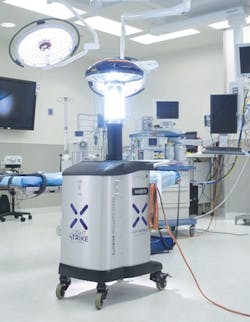
When it comes to using ultraviolet and other light sources to disinfect surfaces and devices, the debate between manufacturers continues and the jury is still undecided about which type of system is best for them and what they can and cannot do to help prevent HAIs.
“UVC kills pathogens effectively in a relatively short period of time. No one disputes that,” said Trapani. “What isn’t clear is whether employing UVC as part of your hospital or nursing home cleaning protocol reduces the spread of pathogens and associated resultant HAI’s. There are several published, peer-reviewed studies that show reductions in HAI’s when UVC is used for disinfection. But many hospitals that have UVC systems have not experienced HAI reductions. Why not?”
Trapani said believing false claims from manufacturers, improper deployment (UVC is not a silver bullet), inability to quantify UVC dose delivery, and lack of real-time disinfection data all contribute to the reason why healthcare facilities aren’t getting the results they expected from UVC.
“Bottom line — understand implementing a UVC system requires extra work, extra cost, a good system with proper delivery measurement and robust real-time reporting, buy-in from various stakeholders, proactive disinfection protocol, and bundling with other interventions,” he said. “The results will save pain and suffering, and will have a net cost savings.”
Trapani provided an example of how Rochester General Hospital health system in NY (which had almost twice the state average C. diff rate) did just that with the RD Rapid Disinfector, improved hand hygiene and antibiotic stewardship.
“At the end of 24 months the four hospitals reported a combined average 20 percent reduction in C. difficile cases, one of the primary pathogens targeted. Leading the initiative was Rochester General Hospital with a stellar 46 percent drop. In the case of RGH alone the results represented a reduction of about 200 C.diff cases out of which many lives have been saved and pain and suffering, and life-changing events eliminated. RGH’s total savings for the period was estimated to be about $6.5 million.”
Chuck Dunn, CEO, President, Tru-D SmartUVC, offered his take on the issues surrounding room disinfection. “Notable researchers in infection control and hospital epidemiology have begun to stress the need for no-touch disinfection technology, which has driven acceptance of UVC robots in prestigious health care systems and hospitals throughout the U.S. ,” said Dunn, who expressed considerable confidence in the work and research of Dr. William A. Rutala who has conducted research using the Tru-D system.
Quoting a passage from a Rutala paper, Effectiveness of Ultraviolet Devices for Terminal Room Decontamination, Dunn said “’Enhanced disinfection technology should be used. If you don’t already have it, you need it in your budgets. Minimally, you must add No Touch Technology to your manual cleaning practices upon discharge of a patient on contact or enteric contact isolation.’”
Dunn suggested roadblocks to implementing the technology might include budget constraints and apprehension regarding workflow and employee downtime. For those who are ready, Dunn offers some information about the Tru-D product: “Tru-D has been validated by more than 15 independent studies, including the only randomized clinical trial on UVC disinfection, which was funded by the Centers for Disease Control and Prevention (the Benefits of Enhanced Terminal Room-Disinfection or “BETR-Disinfection Study”),” he said. “The BETR-D study showed that Tru-D can reduce the risk of acquisition and infection of HAIs including C. diff, MRSA and VRE by a cumulative 30 percent.”
Xenex, another strong player in the room-disinfection market whose product the LightStrike Germ-Zapping Robot operates on pulsed xenon, challenges various claims made by competing UVC disinfection manufacturers.
Ryan Williams, Executive Vice President, Xenex, agrees that market confusion, misleading statements, and other factors have left some at a crossroads when it comes to choosing the right system. He says some companies also take advantage of a loosely regulated category. “Because this market is governed by the EPA, some companies have issued extremely broad statements about the efficacy of their technology,” said Williams — a point of view expressed by most UVC suppliers.
“The only way for a healthcare facility to wade through this confusion when evaluating UVC disinfection — or any type of room disinfection technology — is to make evidence-based decisions,” Williams affirmed. “Not all UVC light is the same. Ask to see the peer-reviewed and published studies from hospitals that saw reductions in their C.diff infection rates, their MRSA infection rates, and their surgical site infection rates. If the vendor can’t show you scientific proof that the technology works, then don’t buy it.”
In response to hospital budget concerns, Xenex says it now offers “The No Risk Infection Rate Reduction Program.” Facilities can use the technology completely free of charge until they achieve specific HAI reductions.
“Ochsner Medical Center on Jefferson Highway, took us up on this, deployed a fleet of LightStrike Germ-Zapping Robots, and saw a 49 percent decrease in infection rates,” said Williams, explaining that Ochsner deployed 10 robots (they now have 16) and monitored its success based on “specific reductions” in C.diff, MRSA and VRE infection rates. “The hospital’s Environmental Services (EVS) team was able to achieve 95 percent utilization of the robots on room discharges and transfers. Data analysis of the first 90 days of the program demonstrated that the hospital experienced a 49 percent reduction in
C.diff infection rates and 49 percent reduction in overall infection rates after adding LightStrike pulsed xenon UV disinfection to its cleaning protocol.”
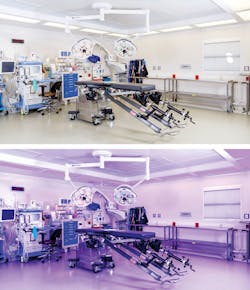
Also available are visible light systems such as the Indigo-Clean Continuous Environmental Disinfection lights which some facilities have installed in the OR, ER, pharmacy, procedure/exam rooms, and patient bathrooms for added, all-day microbial protection.
“By its nature, UV light is highly germicidal, and harmful to humans; it is therefore best used in ‘episodic’ disinfection applications, such as terminal cleaning,” explained Cliff Yahnke, PhD, Director of Clinical Affairs, Indigo-Clean. “Visible light is safe for humans, however, it is less germicidal and is therefore best used for ‘continuous’ disinfection applications. It can also disinfect both hard and soft surfaces, and the surrounding air, which is of interest in orthopedic procedures that can be lengthy, with large open incisions throughout.”
Yahnke said Maury Regional Medical Center in Columbia, TN, installed the lights in an orthopedic operating room for a yearlong study recently published at the AORN conference. Outcomes were notable with a 73 percent reduction in SSIs over approximately 800 cases. “The benefits of Indigo-Clean may also extend beyond the room in which it was installed, as adjacent rooms showed a 75 percent reduction is SSIs as well,” said Yahnke. “Initial measurements suggest that this was due to a reduction in airborne and/or cross-contamination from staff and shared equipment.”
Installation entailed a one-time capital purchase of approximately $20,000 with a much greater return. “By preventing eight infections in the room where it was installed, an estimated $170,000 in costs were avoided based on the national average of approximately $21,000 per SSI.1 “Since it uses LED technology, it is designed to last for 10 years with no replacement parts or consumables required. This suggests that preventing a single infection with Indigo-Clean over its 10-year lifetime would yield a positive ROI.”
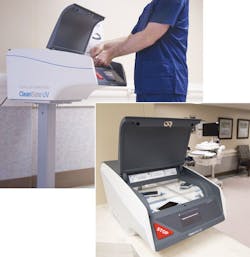
For handheld mobile devices, CleanSlate UV CEO Taylor Mann says his company’s technology can be integrated easily into hand hygiene routines, making it more likely for clinicians to disinfect their devices. “The CleanSlate is often placed next to hand-washing stations, creating a ‘one-stop personal hygiene solution’ where hands and devices are both sanitized,” explained Taylor. “The 30-second cycle allows CleanSlate UV to fit into high-traffic areas without causing congestion, and the touch-free device retrieval prevents recontamination of clean hands when retrieving a device. Our flagship NICU and OR case study saw visitor and staff compliance go from ~10 percent to 72 percent. With JCAHO now scrutinizing how facilities are sanitizing devices — and the effectiveness of those P&Ps — increasing compliance has been a major value driver.” Also important, Taylor said the technology is safe to use, won’t harm devices and therefore will not void device warranties.
Another hot topic
Patient warming has been a hot topic of debate for several years.2 The big question: Do forced-air systems increase risk of SSI or not? In light of some recent declarations made by the Food and Drug Administration (FDA), the argument may start to simmer down. Last August the FDA issued a safety alert on the use of forced-air patient warming products during surgical procedures. “After a thorough review of available data, the FDA has been unable to identify a consistently reported association between the use of forced air thermal regulating systems and surgical site infection,” states the alert. “Therefore, the FDA continues to recommend the use of thermoregulating devices (including forced air thermal regulating systems) for surgical procedures when clinically warranted.”3
There’s also mounting research, including a study published earlier this year by the American Society for Microbiology,4 indicating that bathroom hot-air hand dryers may spread bacterial spores. “Consequently, this study has implications for the control of opportunistic bacterial pathogens and spores in public environments including healthcare settings,” wrote the researchers.”Within a large building, potentially pathogenic bacteria, including bacterial spores, may travel between rooms, and subsequent bacterial/spore deposition by hand dryers are a possible mechanism for spread of infectious bacteria, including spores of potential pathogens if present.”
Air Care
Scientific Air Management’s patented, lab-certified portable air filtration system is another UVC innovation that has helped healthcare facilities treat the air, making it less likely to spread germs. “This is reducing airborne pathogens in a way that’s never been seen before,” said Tom Derrick, Co-Founder & Senior Vice President, OpenMarkets, an equipment research, quoting, budgeting and purchasing
organization.
Mark Schwartz, CHFM, CHC, Director, Medical Center Facilities, University of Rochester Medical Center, NY, shares his experience with the system. “We have deployed these units in the Emergency Department and Trauma Bay to address outside effluents, airborne flu viruses and other pathogens,” said Schwartz. “This effort was met with great praise from both our clinicians and patient population due to increased air quality. The use of the Scientific Air Management units was essential to minimize potential pathogens due to an extremely robust flu season and typically crowded conditions in the Emergency Department.”
“What’s unique and innovative here is that these devices kill the pathogens, as opposed to the more common filter systems,” says Derrick, adding that the system’s UVC light suppression kills airborne pathogens to the 99.9995% level. “HEPA filters don’t demonstrate the same outcomes.”
Decontaminating dirty digits
Moving on to hand hygiene (HH), many healthcare facilities admit to struggling still with compliance, which is difficult to fully understand considering the body of evidence and widespread knowledge of the direct link between HH and HAI rates. There also are many new technologies and hand-washing formulas available to make the task easier and compliant with new regulations.
For example, to comply with FDA’s new ruling on Triclosan, DebMed offers several new antimicrobial and routine soaps to replace Triclosan soaps. “Kindest Kare Advanced Antimicrobial Foam Handwash from DebMed is formulated with 0.50 percent Benzalkonium Chloride, providing healthcare facilities with a phenomenal Triclosan-free soap,” said Ron Chappuis, Vice President, Marketing, DebMed. “Designed with healthcare workers’ hands in mind, Kindest Kare Advanced is a fast acting, antimicrobial soap that is fragrance-free, hypoallergenic, has extensively documented mildness data, and meets both the 1994 and 2015 proposed Health Care Personnel Handwash standards.” He said the products are proven to eliminate 99.999 percent of Acinetobacter baumanni, MRSA, VRE, and CRE in 15 seconds (modified time kill in vitro data).
As for HH surveillance technology, the HPN Infection Prevention survey revealed fewer healthcare providers than expected have adopted the systems, although many did say their facilities are now considering it.
“With any new technology, adoption may take time due to many reasons: the normal uncertainty often found with new technologies, lack of awareness, desire for validation or ‘proof’ that it works and the upfront cost despite proven benefits are a few reasons,” Chappuis said. “There can also be resistance to change, especially in regards to a practice that involves everyone in the hospital — which ironically is why they should be installing these systems.”
Chappuis adds that many HH surveillance systems have been shown to reduce HAIs and increase compliance but are they clinically proven and validated in peer-reviewed journals? The DebMed Hand Hygiene Compliance System meets those standards and has been clinically proven to reduce HAIs by up to 42 percent.
Super sutures
Medical studies and a recent analysis of the research suggest antimicrobial sutures can help prevent SSIs. In the 2017 analysis, researchers looked at 21 randomized clinical trials and of the 138 per 1,000 procedures deemed to place patients at risk of developing SSIs, the use of antimicrobial-coated sutures reduced that risk to just 39.5
“Trial sequential analysis indicates that the effect was robust, and additional data are unlikely to alter the summary effect,” the authors wrote, noting significant cost savings as well. In 2016, the World Health Organization’s Global Guidelines for the Prevention of Surgical Site Infections (SSI) also recommend their use.
Clever catheterization
Until they adopted a solution from BD (Becton, Dickinson and Company), the nurses working in various units at UF Health Jacksonville didn’t have a standard in place for peripheral vascular access, which led to several problems. “There were frequent missed starts on first attempts to insert peripheral catheters, which required additional attempts that caused patients additional discomfort and wasted resources,” said BD Spokesperson Matt Coppola. “In addition, the legacy peripheral catheters did not have an integrated extension set, which added to variability. Nurses had to remove the IV dressing to attach an extension set when patients were moved from the emergency department to the floors. Every change raised the risks of blood exposure and potential IV dislodgement. The hospital serves a large population of HIV- and Hepatitis C-positive patients, so protection against blood exposure is critical.” These issues became the basis of a recent UF Health Jacksonville case study.6
Coppola said BD’s clinical team helped the hospital develop process improvement projects that aligned with best practices, which included using BD’s vascular access management (VAM) integrated solution. “The integrated solution from BD for vascular access management provided specific and practical recommendations, including policy updates and practice changes, extensive training on best practices for PIV insertion, and product recommendations,” he said. “The UF Health Jacksonville “Nurse Safety Team” also worked with BD to find ways to decrease blood contamination, increase safety during the placement of intravenous catheters, decrease blood culture contamination, and review the opportunities to decrease central line-associated bloodstream infection (CLABSI) by improving access and techniques used in IV access.”
After one year UF Health Jacksonville had a zero incidence of blood spillage and contamination, a near 50 percent increase in first-stick proficiency, average PIV dwell time increased from 2.4 days to 4.3 days, decreased catheter use, and a 46 percent decrease in catheter replacement within 48 hours.
Pat Parks, MD, PhD, Medical Director, 3M Critical and Chronic Care Solutions Division, adds that PIV-associated bloodstream infections (BSIs) are not regularly monitored or reported even though PIV procedures are ubiquitous in healthcare facilities. “Nearly every patient admitted to a hospital will have a PIV line, presenting a potential entry site for infection causing bacteria,” Parks said.
Mercy Hospital, a 900-plus bed, not-for-profit Level I trauma center in St. Louis, MO, tried for several years to reduce BSIs but its biggest success came after adopting a comprehensive PIV catheter maintenance bundle, which included consistent disinfection of needleless connectors and male luers and use of 3M’s Curos disinfecting caps for needleless connectors. “They contain a 70 percent isopropyl alcohol (IPA) which can disinfect against a number of microorganisms commonly associated with BSIs in less than a minute and provide protection for up to seven days, if not removed,” parks said. “The Curos Tips disinfecting cap strips also contain 70 percent IPA and easily twist onto most male luers to help reduce the risk of contaminants from entering the IV line. The unique design limits excess alcohol from entering the lumen while ensuring adequate alcohol is exposed to the exterior surface.
“Mercy Hospital was able to achieve 89 percent compliance among staff using Curos disinfecting caps on PIV lines and 84 percent for Curos Tips disinfecting caps on all disconnected IV tubing,” continued Parks. He noted study results recently published in The Journal of the Association of Vascular Access show that using a comprehensive PIV maintenance bundle, including consistent disinfection of needleless connectors and male luers, reduced microbial contamination of IV access points.”
Nare care
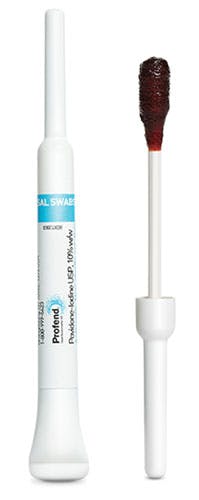
Bacterial colonization in the nose is also a growing concern among clinicians looking for ways to defend against microbes, particularly since the anterior nares are the primary reservoir for S. aureus carriage. “Up to 30 percent of healthy adults are nasally colonized, and with at least 42 percent of S. aureus bacteria resistant to Methicillin, nasal decolonization has proven a significant part of infection control and prevention programs,” said Paul Kelleher, Senior Product Manager, Interventional Care, PDI. “However, compliance has proved to be an issue, because of negative patient experiences, complicated and messy applications and continued reliance on antibiotics.”
Profend Nasal Decolonization Kit from PDI aims to address those issues. “In a product study, the Profend product was found to kill 99.9 percent of S. aureus bacteria after one application, where a similar product only killed 82 percent of the bacteria after three applications,” said Kelleher. “A clinician study of the Profend product found that more than 90 percent of clinicians preferred the Profend product to other nasal PVP-iodine products, highlighting its easy, mess-free application and intuitive design as core competencies that would ultimately increase the likelihood of compliance.”
Mindful monitoring
Keeping a close watch on patients is also fundamental to preventing the development or worsening of HAIs. Today’s advanced technologies are helping clinicians to do that in ways that older platforms can’t. The Amplion care assurance platform, designed to replace legacy nurse call systems, combines advanced nurse call, care collaboration tools, alarm management, reporting and data analytics in a single system to track, manage and confirm care delivery for every patient.
“More importantly,” said Cathy Swenson, RN, Client Success Manager, Amplion Clinical Communications, “it closes care loops, promotes teamwork and supplies the data analytics nursing leaders need to provide safer, smarter patient care and a better patient experience that yields higher HCAHPS scores and lower costs.
“Round and Turn reminders/assurance from the Amplion Alert system help the nursing staff ensure the patient is checked every hour and turned every two [hours],” she continued. “Effectively rounding reduces time patients may lay in bed soiled which could potentially impact or create wounds that become infected. Turn reminders help to prevent skin breakdown which creates an environment for wounds and infections.”
Tainted textiles
Healthcare textiles also have a strong potential to spread pathogens and one type in particular is getting a lot more attention in recent years — mattresses. Most hospital mattresses today are made with breathable fabrics that wick away perspiration, but this means they also absorb a lot more disinfectant during dwell time. This can cause a more rapid deterioration compared to older vinyl mattress skins, which means a lot more places for pathogens to colonize. In fact, mattresses and covers were No. 3 on ECRI’s Top 10 Hazards last year.6 “Bed and stretcher mattresses can remain contaminated after cleaning, putting patients and staff at risk of exposure to body fluids or microbiological contaminants,” ECRI stated. “Reported incidents include patients lying on an apparently clean bed or stretcher when blood from a previous patient oozed out of the support surface onto the patient.”
Bruce Rippe, CEO, Trinity Guardion, says the FDA classifies hospital beds as either Class I or Class II medical devices, which is an important distinction because of their stringent cleaning and disinfection requirements. For example, last year the Centers for Medicare & Medicaid Services (CMS) required following the instructions for use (IFU) on all medical devices and disinfection products. But when it comes to keeping hospital mattresses safe for patient use and in good condition that’s a tough assignment.
“Current FDA reprocessing guidelines requires device manufacturers to prove efficacy of the cleaning and disinfection process of hospital mattresses using compatible disinfectants,” said Rippe. “When disinfectants that are not compatible with the fabric are used regularly, they break down the polyurethane coating and will eventually result in leaks of contaminated fluids into the mattress core, which is defined as mattress failure. The surface energy of the mattress fabric also changes after repeated disinfection cycles, which requires more rewetting to maintain the disinfectant’s required wet dwell time on the mattress surface.”
He says mattress manufacturers used to tell customers to follow their own facility protocol for bed disinfection. However, today many mattress manufacturer IFUs instruct facilities to engage in a multi-step disinfection process to preserve mattress integrity while maintaining FDA requirements. “These new processes include pre-cleaning, cleaning, disinfecting, rinsing and drying steps,” Rippe said. “In addition, some manufacturers have significantly shortened the stated expected useful life of their expensive mattresses due to the expected deterioration of the mattress fabric, and require periodic and per-patient inspections by environmental services staff.”
Trinity Guardion offers a solution: “The Soteria Bed Barrier is a mattress, bed deck and pillow barrier with a simple, compliant disinfection protocol,” said Rippe. “When used as instructed, the Soteria process helps protect new patients from the bioburden of previous bed occupants, preserve the integrity of mattresses, and defend hospital reputations and budgets by reducing bed contamination and related infection risk and consequences. Each is uniquely barcoded for tracking, inspection and documentation, and can be reused up to 150 times.”
Patient privacy curtains can also lead to the spread of HAIs, especially when not changed and handled properly. For facilities that do prefer to use privacy curtains, new options exist to make removal and cleaning fast and easy. Amy Hewett, Infection Prevention and Employee Health, Pender Memorial Hospital, NC, says the IPs and EVS staff using Prime Medical’s Chlorine-Shield Privacy Curtains and are very pleased with the various features they offer. “We totally love the curtains,” Hewett said. “Recently we had a busy night with a trauma and blood was everywhere. All we had to do was snap off the soiled panel and snap on a new panel. The one thing we didn’t need that moment was a ladder in the room. As Employee Health Coordinator, ladder falls are an issue. It’s nice to have a product that’s easy to use, protects our patients and removes the ladder.”
Chlorine-Shield Privacy Curtains have a modular design with a mesh top that attaches to an existing ceiling track and the antimicrobial privacy panels can be removed and replaced without a ladder or equipment — in less than one minute, according to Wendy Brady, Vice President, Marketing, Prime Medical. “The 6-foot privacy panels overlap to accommodate any room configuration and are easily changed by a single staff member when visibly soiled, after contact isolation precautions, at terminal cleaning or for general wash schedules.”
Brady said the panels feature a patented technology that binds chlorine molecules to the fabric when laundered in EPA-registered bleach. “The chlorine-shielded panels resist the colonization of bacteria and viruses, and can be recharged for a lifetime of antimicrobial effectiveness,” she said, noting that a clinical trial showed the curtains had an 81 percent reduction of bioburden when compared to non-treated curtains. “Cost-effective and easy-to-use, Chlorine-Shield Privacy Curtains offer long-term ROI, reliable antimicrobial protection and an environmentally conscious choice over disposables.”
It also matters what healthcare workers are using to clean hard surfaces. Microfiber has become popular but there is concern about laundered vs. single-use varieties, as Warwick Spencer, Business Manager, Contec, explains. “Unfortunately, laundered microfiber mops and wipes have been determined to add to — rather than reduce — this risk,” he said. “ATP testing results show laundered microfiber mops and wipes don’t clean as well as new ones. They are engineered to effectively grab and remove bioburden from hospital floors or surfaces; however, that same technology keeps them from letting go of it even in the best of laundering processes. Therefore, each time microfiber mops and wipes are reused, facilities run the risk of re-depositing dangerous pathogens back into patient critical areas, exposing them to infection. In addition, the built-up residues neutralize disinfectants, rendering them ineffective in eliminating the pathogens that endanger vulnerable patients.”
Spencer says one facility that switched to Contec’s Premira single-use microfiber mops and wipes experienced notable improvements in several areas. “Contec’s client, who previously oversaw an in-house laundering system, summed the experience up by saying, ‘Infection rates, room turnover time, and burnishing rates are all notably down since the transition.’”
Cross-check for clean
Residual bioburden on medical devices can be nearly impossible to see with the naked eye, and even worse is when bacteria get lodged inside endoscope channels. That’s why it’s critical to remember the popular phrase “if it’s not clean it can’t be sterilized” and take action to ensure instruments are free of debris. To help get the job done Healthmark Industries just announced in May the arrival of its FIS-005SK, the next generation of flexible inspection scopes to its ProSys Optical Inspection product line.
“The design and look are different, but also from an infection prevention standpoint, the video and image capture has a much clearer quality than the previous model,” said Healthmark Marketing Manager Matt Smith. According to details provided by Smith, the scope handle has a distal tip composed of a light source and camera lens at the end of a 110cm flexible blue shaft with white graduation marks. Manufactured for instruments 2.0mm in diameter or larger, the FIS-005 is ideal for getting a visualization of any potentially soiled or damaged item. Starter Kit Software is included, which installs on Windows 7, 8 and 10 PCs. This powers the FIS-005 via USB and allows for viewing, recording and documentation.
Case Medical’s universal Case Soil Indicator is another innovation to help ensure manual and/or automated washing is successful. “Existing wash indicators on the market are designed primarily to monitor protein residuals or the effectiveness of alkaline cleaners. Plastic substrates are not truly representative of surgical devices and protein-only indicators are not indicative of the variety of soils remaining on used surgical tools,” said Marcia Frieze, CEO, Case Medical. “Studies indicate that the presence of protein residuals contribute to corrosion of stainless steel instruments. Carbohydrates and lipids may promote adhesion and even growth of bacteria on device surfaces. Using an indicator that is more representative of soiled devices they are trying to clean is a better option for infection prevention.
“Case Soil Indicators are not specific to a washer or chemistry and encompass all elements of a soiled surgical instrument in a representative tray,” continued Frieze. “While other indicators may be useful for invasive surgical procedures, where blood is primarily present, Case Soil contains sterile blood components as well as other representative soils, such as fats and carbohydrates for monitoring minimally invasive, endoscopic devices.”
References
- Zimlichman E., et al. “Health Care-Associated Infections: A Meta-analysis of Costs and Financial Impact on the US Health Care System”, JAMA Intern Med, 173; 2039-46 (2013).
- https://www.hpnonline.com/3m-responds-to-patient-warming-heats-up/
- https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm574053.htm
- http://aem.asm.org/content/84/8/e00044-18.abstract
- https://onlinelibrary.wiley.com/doi/abs/10.1002/bjs.10445
- https://www.BD.com/en-us/offerings/integrated-solutions/vascular-access-management/vascular-access-management-for-peripheral-iv-care
- https://academic.oup.com/femsle/article/363/21/fnw236/2681730
2018 Infection Prevention Buyers Guide
Vendor listings and product spotlights

Valerie J. Dimond | Managing Editor
Valerie J. Dimond was previously Managing Editor of Healthcare Purchasing News.