In an age of increasing antibiotic resistance and healthcare-associated infections (HAIs), clinicians are eager to diagnose and treat their patients correctly and quickly. In response, researchers and manufacturers are working diligently to deliver infection screening tools that work faster and more accurately than those of earlier generations. Diagnosing and treating the flu, for example, takes just minutes when using the latest point-of-care (POC) testing tools. Considering that some clinicians have mistakenly treated the flu virus as a bacterial problem, being able to perform a rapid, accurate test at the bedside is a big achievement in the fight against antibiotic resistance.
Fighting flu faster
Clinicians can diagnose respiratory illnesses either via observation, rapid antigen testing, traditional lab testing or molecular point-of-care testing, said Chris Newhouse, PhD, Group Marketing Manager, Microbiology, Roche Diagnostics Corporation. “Accuracy is a concern with both observation alone and point-of-care rapid antigen tests, and sending a negative sample out to have a lab confirm the results may delay treatment 24 to 48 hours or more,” said Newhouse. “Traditional culture testing in a lab can take 24 to 48 hours, and lab-based respiratory panel tests can be costly and often include unnecessary testing.”
When it comes to caring for vulnerable inpatients, such as those in the pediatric emergency department (ED) with respiratory virus, molecular POC testing is the ideal solution, according to one study published in the Journal of Molecular Diagnostics. “Of 61 ED-initiated tests, physicians indicated in 39 cases, or 64 percent, they would change patient management if bedside viral results were available,” Newhouse said. “The authors concluded that PCR-based rapid bedside molecular point-of-care testing for acute respiratory illnesses has the potential to decrease ED length of stay, reduce diagnostic tests and patient charges, and increase appropriate use of antibiotics and antiviral agents. Being able to reduce the length of stay in the ED for patients infected with RSV also has the infection control benefit of getting infected patients out of the ED faster; reducing the time they might be exposing other ED patients to RSV.”
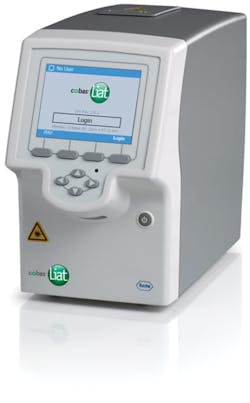
Roche’s cobas Liat PCR System delivers lab-quality, real-time polymerase chain reaction (PCR) molecular testing with CLIA-waived tests for strep A, flu A/B, and flu A/B plus RSV. The test takes one minute to perform with definitive results available in 20 minutes or less. No confirmation is needed giving clinicians the ability to provide immediate treatment. Another study in the Journal of Clinical Microbiology that compared Roche’s cobas Liat PCR System to a lab reference standard showed no difference in sensitivity and specificity between the two tests for either flu A or B. “Providing greater certainty about whether antibiotics are needed and ensuring the right medication is delivered can lead to more appropriate antibiotic use—which is the heart of the antibiotic stewardship philosophy.”
Also important to note is information provided in the Centers for Disease Control’s (CDCs) “Guidance for Clinicians on the Use of RT-PCR and Other Molecular Assays for Diagnosis of Influenza Virus Infection.” This states that some molecular assays will detect influenza A or B viruses but not determine the influenza A virus subtype. If the subtype is identified that would indicate the presence of a novel influenza A virus.1 Novel influenza A viruses are usually transmitted from avian or swine species to humans.
Conversely, some FDA-cleared devices can detect some or all of the seasonal influenza A virus subtypes, stating: “These assays will not only identify the currently circulating influenza A virus strains, but also may identify viruses that are detected as influenza A for which no subtype could be identified. These ‘unsubtypables’ may represent novel influenza A virus infections. Clinicians and laboratorians using molecular assays that are capable of detecting all currently circulating seasonal influenza A virus subtypes [i.e., A(H1N1)pdm09 or A(H3N2)], and who identify an “unsubtypable” result (i.e., influenza A with no subtype detected), should contact their state or local public health laboratory immediately for additional testing to determine if the infection is due to a novel influenza A virus.”
Surveillance as a sepsis solution
Inpatient deaths from sepsis — five million annually around the world — have increased, although the rate of incidence has changed little over the last five years. “Despite the increased visibility of sepsis and increasing awareness of the global health challenge it poses, accurate quantification of sepsis incidence, mortality and morbidity remains elusive,” wrote medical researchers in an October 2017 issue of the Journal of the American Medical Association.2 “In the absence of a definitive diagnostic test for sepsis, the gold standard remains the clinical diagnosis of infection and identifying new organ dysfunction caused by the host response to that infection.”
Sean Benson, Vice President and General Manager of Specialized Surveillance, Wolters Kluwer Health, says early diagnoses can be difficult to achieve. “One issue is that initial symptoms mimic several common illnesses. But early identification is also hampered by difficulties aggregating and analyzing siloed data to identify at-risk patients and communicate actionable recommendations to frontline clinicians,” said Benson.
One tool by Wolters Kluwer, the POC Advisor, offers an innovative solution that can help clinicians stop sepsis in its tracks. The device collects a variety of information from numerous data sets, analyzes it and, if warranted, generates an immediate sepsis warning alert so that clinicians can intervene and provide patients with appropriate treatment right away. Benson explained that the clinical intelligence platform “aggregates and normalizes patient data from disparate clinical systems, which is automatically analyzed using hundreds of rules that also account for comorbid acute infections, medication abnormalities and chronic diseases to create patient-specific alerting. The rules engine also integrates evidence-based clinical content from UpToDate, Lippincott and Lexicomp, and guidance from ProVation Order Sets. Thus, when predictive analytics indicate sepsis, clinicians are immediately alerted and provided with evidence-based treatment advice.”
Benson also claims that the POC Advisor “is the only sepsis solution scientifically proven to improve outcomes,” which he supports with a study published in the Journal of American Medical Informatics Association.3 In this study the tool was compared to gold standard physician chart reviews. “The [POC Advisor] platform achieved unmatched alert sensitivity (95 percent) and specificity (82 percent), reduced sepsis mortality by 53 percent and related 30-day readmissions by 30 percent, and lowered length of stay,” he said. “By accelerating detection of sepsis, clinicians can begin appropriate treatment at its earliest stages. By avoiding the need for critical care, POC Advisor may also reduce the risk that a sepsis patient will develop post-sepsis syndrome.
“At 48 percent, sepsis is the inpatient service with the highest growth projection from 2020-2025,” continued Benson. “This will exacerbate the heavy financial burden already borne by hospitals, thanks to Medicare reimbursements that are typically less than half the costs of treatment. To address this looming crisis, The Advisory Board recommended reinforcing nurse-led sepsis protocols by embedding a screening tool into the triage process, and by simplifying the complex sepsis protocol to prevent treatment delays. Doing so has the potential to save $2 million annually for a typical 300-bed hospital, $5 million for a five-hospital system, and $7.5 million for a regional system.”
Bucking bloodstream infections
You might not think an IV securement dressing could be part of a solution for preventing bloodstream infections but some models do actually help clinicians prevent or provide early treatment for them.
“Bloodstream infections are some of the deadliest and costliest hospital-acquired infections — yet are largely preventable,” said Pat Parks, MD, PhD, Medical Director, 3M Critical and Chronic Care Solutions Division. “To identify infections, clinicians look for common symptoms such as a fever that occurs in the absence of another infection and redness or soreness around the insertion site. Some IV dressings block visibility to the insertion site, making monitoring for early signs of infection difficult. Frequent dressing changes to monitor the insertion site can be disruptive to patient care and increase the patient’s risk of infection. In fact, one study demonstrated that more than two unscheduled dressing changes can increase the risk of infection 10 times.
“To combat these hurdles, 3M Tegaderm CHG IV Securement Dressings provide clinicians continuous site visibility to help reduce the number of unscheduled dressing changes and quickly identify potential complications,” Parks continued. “Over the past four decades, 3M has continuously innovated Tegaderm Dressings, including adding advanced securement and antimicrobial protection capabilities. 3M Tegaderm CHG IV Securement Dressing is the only transparent dressing indicated and proven to reduce catheter-related bloodstream infections (CRBSIs), aligning with evidence-based guidelines and practice standards from the CDC and Infusion Nursing Society.”
Looking ahead
At Wolters Kluwer Benson says the POC Advisor early response module will be expanded for identifying other life-threatening, rapidly-deteriorating conditions such as pulmonary embolism, pneumonia, COPD, dysrhythmias, hemorrhage, heart failure, stroke, hypoxia and renal failure.
“These are among the most complex and costly conditions to diagnose and treat,” he said. “As with sepsis, the POC Advisor Rapid Response Module will leverage its powerful rules engine, early and accurate alerts, and prescriptive advice to detect the early warning signs that can signal the onset of these conditions and alert rapid response teams to intervene before the patient becomes critical.”
Newhouse (Roche) said the company is planning additional cobas Liat PCR tests for MRSA plus Staph aureus, and C. difficile pertussis (whooping cough), and others.
Roche currently offers an automated lab-based PCR system for the HAIs which can be useful as each play an important role in helping facilities to optimize their testing resources based on patient type and urgency.
“For example, a September 2017 article4 describes how HealthPartners in Bloomington, MN, developed an integrated strategy for C. difficile testing to address the varying inpatient and outpatient needs of the health system, all with the common goals of timely treatment and prevention, as well as cost efficiency,” explained Benson. “The HealthPartners system includes both inpatient and outpatient testing, and the author, Dr. Nicole Khoury, who is the Laboratory Scientific and Accreditation Director, explained that their hospitals use PCR for C. difficile testing, and a one-hour turnaround time is paramount both for care of CDI patients and infection control.
However, inpatient testing is only a part of the overall CDI testing need. About 5,000
C. difficile test orders per year also arrive from their outpatient clinics. Their outpatient CDI testing shares the need with inpatient testing for performance—sensitivity and specificity—and an easy-to-use system. However, the turnaround time expectation for outpatient is less stringent; same-day results are adequate for patient care needs.”
References:
- https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm
- https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm
- https://academic.oup.com/jamia/article/24/1/88/2631454
- https://www.mlo-online.com/diagnostics/assays/article/13009259/optimizing-c-difficile-testing-in-a-large-system-lab-by-integrating-inpatient-and-outpatient-needs

Valerie J. Dimond | Managing Editor
Valerie J. Dimond was previously Managing Editor of Healthcare Purchasing News.