Healthcare-associated infections (HAIs) just won’t go away — or will they? On a national level, catheter-associated urinary tract infection (CAUTI) rates remain relatively unchanged. But on the state level, according to the Centers for Disease Control’s (CDC’s) latest progress report, 18 states performed better at preventing CAUTI than the rest of the country. Central line-associated bloodstream infections (CLABSI) are also down 50 percent, a four-percent improvement from the previous year. In fact, CLABSI rates have been decreasing for several years.
If some facilities can achieve consistently low infection rates — primarily by adhering to evidence-based practices, adopting new technology, and choosing infection prevention (IP) products wisely — isn’t it time for all healthcare providers to do the same?
Think outside the box
According to Gregory D. Wiita, President and CEO, Poiesis Medical, most facilities are reluctant to change, despite the available evidence that their treatment methods may fall short. “The basic bundles being used today existed over 20 years ago,” said Wiita. “The feedback from customers directly is they are following the bundles, so they are doing their job and checking the box as to whether the ‘CAUTI bundle’ is being followed. If yes, they move on, while rates may stay the same,” Wiita noted. “The why any facility would not be extremely concerned with high CAUTI rates is squarely based on CMS metrics. The methodology behind the predicted number and the standard infection ratio (SIR) affords facilities a false sense of progress. Offering best care practices is to aim for zero by embracing protocols and products that can assist in this goal. Seems we are reaching a point where healthcare employees may have CAUTI fatigue, they become frozen to change, afraid to make a move. We look to the major thought leaders as a cause; by not engaging innovative companies and updating the bundles, nothing is going to change.”
For example, Wiita says indwelling single-balloon urethral catheters (Foley catheters) are one of the leading causes of CAUTI, yet the clinical need for the right medical indication is vital. He referred to research1,2 that shows Foley’s cause urethral irritation and damage to the protective mucosal lining of the bladder, from the catheter tip, and bladder spasms that can disrupt the position of the anchor balloon, allowing bacteria to attach to the wall and colonize. These and other problems attributed to Foley use weaken the bladder’s natural ability to fight infection. However, they’re still the standard in most facilities.
“’We’ve always done it this way’ exists in many facilities both for infection prevention and other initiatives,” said Timothy Bowers, MT(ASCP) MS, CIC, FAPIC, Director, Infection Prevention, Inspira Health Network, Vineland, NJ. “While that practice was instituted for a good reason, it shouldn’t be a barrier to improving. Approaches should be tailored to the problem that is causing the infection. Good improvement processes let the data guide your team to an approach that will make the most impact on the outcome. If contamination occurs while the catheter is inserted, then work on that aspect of care.”
Bowers, who is also a Communications Committee member at the Association for Professionals in Infection Control and Epidemiology (APIC), believes good, consistent hand hygiene and general cleanliness are the “backbone” to infection prevention but, in some circumstances, adding new, innovative healthcare products can be equally as important. “While I stay neutral about specific products, over time industry has shown to adapt to the needs of our patients time and again,” said Bowers.
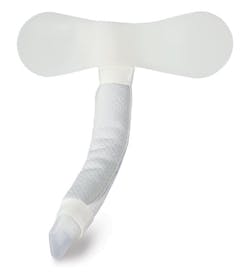
The Duette Urinary Drainage System by Poiesis may support that sentiment. It features a bladder protection balloon encapsulating the catheter tip. The second balloon can prevent bladder damage. Also, the drainage eyes are located between the balloons to prevent the bladder wall from being aspirated into the eyes. By reducing fluid turnover the innate immune defense of the bladder is comprised. The Duette is about $2.00 more per unit compared to Foleys but facilities that use them think it’s worth it. “A leading Southeast teaching and Level one trauma facility has been using the Duette for over three years in the NSICU ward where our pivotal study took place and now is house-wide for over two years,” said Wiita. “If facilities were not experiencing value-based metrics they would have stopped using the product — 35 hospitals — are experiencing durable results of over 50 percent reductions. If the healthcare industry is going to align with HHS’ new targets of HAI reduction by 2020, new tools and fresh thinking on the CAUTI topic will be required. Changing the standard of care to a patient centric product is the goal.”
Place it on the outside
Clinical guidelines indicate that indwelling catheters are risky, should only be used when absolutely necessary, and removed as soon as possible to avoid infection. For some patients, an external urine collection device is a great option.
“Many of the available options for urinary incontinence management are archaic and/or invasive,” said James Harders, PhD, Director of Engineering, BioDerm, Inc. “When it comes to issues such as nosocomial-related injuries or infections, Men’s Liberty is unique in that it is totally external and makes no invasive contact to the human native tissue. The device can be easily attached and detached painlessly on ridged or flaccid tissue, which is difficult for condom-style catheters and tube-style catheters which have been known to cause pain during application, use and removal. The same product may be used at the hospital and at home, either the Men’s Liberty Acute or the Men’s Liberty, though the needs of the patient will drive which device best meets their needs.”
Until recently, external options were only available for men. Luckily, that’s changing.
“In this particular case, with the increased focus on reducing CAUTI across the country and in response to the male external catheters, there are now product-based alternative solutions to indwelling urinary catheters in female patients too,” said Bowers.
Sage Products LLC, now a part of Stryker, offers an external collection device (ECD) called PrimaFit for female patients.
“Several ECDs or non-invasive devices that divert and contain urine already exist for the male patient population,” said Mike Nygren, Director of Marketing Communications for Sage. “However, a viable and effective ECD for the female population had yet to be developed due to the complexities of the female anatomy. For an external urine collection device to be effective, it needs to fit the female patient’s anatomy and stay in place as the patient moves. The ability to contour the device to the patient’s anatomy provides reassurance that it is properly positioned for optimal performance. If it is not secured to the patient, the device could migrate down and underneath the patient, increasing the risk of a device-related pressure injury as the patient moves around or is repositioned in bed.
“PrimaFit is an innovative external urine management system that can serve as a transition from indwelling catheters to independent continence by promoting early catheter removal or avoid catheter use altogether,” Nygren explained. “The dual secure design and flex-fit core help keep the device comfortably and properly positioned while the soft, woven fabric wicks urine away from the skin, helping address a risk factor of incontinence-associated dermatitis. Urine is then suctioned from the tapered end cap into a collection canister. The ability to contour and secure the Sage Female External Urine Management System to the patient’s anatomy allows the device to accommodate most of the female patient population, regardless of age or body size.”
Sarah Skelton, Senior Product Manager, Bard Medical Division, notes that un-catheterized female patients who are bedridden and/or incontinent are no longer limited to absorbent pads or diapers which can cause discomfort and skin damage.
“The BARD PUREWICK Female External Catheter addresses this clinical challenge by providing a simple, noninvasive option for urine output management; the PUREWICK Female External Catheter helps reduce urinary catheter days, lowering CAUTI risk, and protects skin by wicking away urine,” asserted Skelton.
So will these types of external female products become standard practice for avoiding CAUTI? Time will tell.
“With the tremendous amount of focus and effort placed on reducing CAUTIs nationwide, significant clinical evidence is necessary to substantiate claims regarding CAUTI reduction,” Skelton said. “Due to the novelty of female external catheters within the market, peer-reviewed clinical evidence has not yet been produced. However, the PUREWICK Female External Catheter does provide a new, non-invasive option for urine management when an indwelling catheter is unnecessary or no longer needed. This allows institutions to continue to reduce catheter dwell time and ultimately CAUTI risk. Hospitals can set policy regarding which patients receive an indwelling catheter, but one factor that is out of their control is patient gender. Female patients are more likely to have an indwelling Foley catheter and are at a three times higher risk for developing a CAUTI. The PUREWICK Female External Catheter provides a new non-indwelling management option for women.”
“Every patient is different; no cookie cutter models and there will not be a one size fits all solution,” said Vicki G. Allen MSN, RN, CIC, FAPIC, Infection Prevention Director, CaroMont Regional Medical Center, Gastonia, NC and APIC Communications Committee member. “This is when a committee with multiple players can be effective in an effort to determine the appropriateness, necessity, and costs of products.”
Bye-bye blood stream infections
On the whole, facilities are more successful at battling blood stream infections (BSIs), especially CLABSI which, as mentioned earlier, have been in steady decline for years. But the goal, ultimately, is to reduce the BSI rate to zero, meaning more effort is required.
For example, catheter hubs (entry ports) are perfect thruways for bacteria to travel and one of the leading causes of BSIs if not properly disinfected when accessed. “Adequately scrubbing the hub depends on the agent you use, appropriate contact and drying time, and—most important—friction,” states The Joint Commission’s CLABSI Toolkit. However, complying is sometimes a challenge for busy clinicians and the varying standards can also cause confusion.
Christie Chapman RN, BSN, CIC — IPCE III, UCSD Health, Infection Prevention and Clinical Epidemiology, says variability plays a strong role. “The recommended length of time to ‘scrub the hub’ to adequately remove microbes from the port site varies between research — as widely as five to 60 seconds,” she said. “Do healthcare personnel scrub long enough (and with enough friction) to remove large microbial contamination from the hub? You can’t see microbes, how do you know how contaminated the hub is? There is also some variability on the appropriate antiseptic. CDC/HICPAC recommends CHG, providone iodine, an iodophor or 70 percent alcohol.3
“Then there are the human factors; humans make mistakes,” continued Chapman, who sits on the Board of Directors at the Association for Vascular Access (AVA). “Healthcare personnel get busy, have their hands full and even simply forget to scrub the hub before injecting. I was reviewing a conversation on a nursing blog site recently around scrubbing the hub before medication injection and RNs were very honest that though they know they are supposed to scrub the hub, they are human and do forget [to do this] important step. In a study by Lee in 2013, the disinfection compliance by clinicians prior to administering medication through an access port was only 10 percent.4 Despite the 1,000 things a conscientious healthcare provider does right for their patients every day, this common break in aseptic technique can set a patient up for a potentially life threatening BSI.”
Pat Parks, MD, PhD, Medical Director, 3M Critical and Chronic Care Solutions Division, adds that even if clinicians think they’ve scrubbed properly there’s no real way to identify whether disinfection was complete. “The scrub the hub technique makes it more difficult to maintain and measure compliance as there is no visible evidence that the IV access point has been disinfected,” said Parks. “Employing passive disinfection methods such as disinfecting port protectors can help clinicians avoid many of the problems associated with the scrub the hub protocol.”
Solutions are available to help address port contamination so that facilities don’t have to rely on hub-scrubbing alone, if at all.
“3M Curos Disinfecting Port Protectors provides consistent disinfection and a visual indication to care providers that the hub has been disinfected,” said Parks. “3M Curos Disinfecting Port Protectors easily twist on to a variety of needleless connectors, providing continuous passive disinfection and protection to these IV access points for up to seven days. Furthermore, they improve compliance because the bright color cap offers a visual cue that staff is following disinfecting procedures.
“A growing body of clinical evidence, including systematic review and meta-analysis, supports that using 3M Curos Disinfecting Caps for Needleless Connectors and 3M Curos Tips Disinfecting Cap Strip for Male Luers can help ensure proper maintenance and industry standard compliance,” continued Parks. “Another published study showed one hospital decreased its CLABSI rates by more than 40 percent after implementing 3M Curos Disinfecting Caps, resulting in $300,000 in estimated annual savings.”5
Steve Bierman, MD, Chief Medical Officer, Access Scientific, believes clinicians, in general, still have to work harder than necessary to get the most effective products for their vascular patients. “Clinicians struggle with the limitations placed on their choices: specifically, the best (in terms of scientifically proven) medical devices are not always the devices allowed into an institution by the supply chain specialists,” Bierman said. “Given the right array of alternatives, and full unalloyed information, clinicians will almost always make the right choice.”
He says Access Scientific’s POWERWAND vascular devices have been studied extensively in recent years, including five published peer-reviewed studies and another one on the way that show zero bloodstream infections occurred over the course of 20,000 catheter days when using POWERWAND products.
“In a study out of Richmond Medical Center, published in 2015, a single ventilator ICU experienced eight CLABSIs prior to the POWERWAND midline being introduced,” Bierman said. “After POWERWAND introduction, central line catheter days were reduced by 37 percent — doctors removed CVCs faster because they trusted the POWERWAND — resulting in a sustained 100 percent CLABSI reduction. The POWERWAND has resulted in similar reductions in central line utilization and in CLABSI throughout the country. In fact, these reductions are so consistent that Access Scientific offers a risk sharing agreement to our customers which guarantees this kind of outcome.”
Stopcock contamination stops here
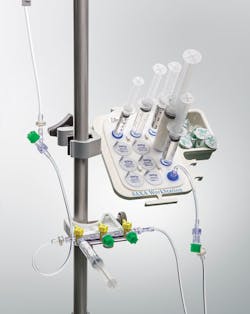
Stopcock handling in the anesthesia area is yet another source of infection that facilities need to address. “Several published studies have demonstrated contamination in the anesthesia work environment, including syringes, vials, and clinicians’ hands,” explained Steve Weber, Director of Marketing, IV and Vascular Access Systems, B. Braun Medical Inc. “Randy Loftus, MD, has published extensively about this topic and a recent publication confirms the relationship between anesthesia work environment contamination and bacterial transmission through the open lumen of the stopcock.”
B. Braun’s SAXA Anesthesia Disinfection Workstation provides a convenient and helpful solution for conducting effective decontamination activities. The workstation features the DOCit IPA device for syringe and male luer tip disinfection and the Hubscrub IPA device for disinfecting stopcocks, needleless connectors, and other female luers. The workstation also keeps syringe and other devices neat and organized and it has a slot for a hand hygiene dispenser.
“Both the DOCit and Hubscrub devices contain 70 percent isopropyl alcohol in the cap and allow for appropriate disinfecting in 10-seconds due to the unique internal designs that scrub the contact surface,” said Weber, noting a 2012 study showing use of the workstation helped reduce open lumen bacterial contamination significantly.
Augmenting with antimicrobials
Lise Moloney, Director of Business Development — Healthcare, Sciessent LLC, discussed how the company’s Agion antimicrobial, embedded directly into the polymers that are used to make medical devices, offers added protection.
“Every surface and every crevice contains the antimicrobial; this is not a surface treatment so there are no concerns of surface coatings wearing off or uniformity of a coating,” Moloney explained. “We look at the use of embedded antimicrobials as a second line of defense, similar to airbags in cars. Take Agion-treated central venous catheters as an example. Clinicians still need to follow best practices for insertion and maintenance of the catheter, but if there are lapses in best practices or if compliance is low, the Agion-treated catheter provides a second line of defense against microbial contamination.”
The Agion-treated catheters are inserted just like any other catheters so there’s no learning curve or additional training required. Moloney said studies of Agion-treated central venous catheters show a 90 percent reduction in catheter-related bloodstream infections (CRBSI) compared to a standard central venous catheter.6 “This study looked at the use of Agion-treated catheters in a high risk patient population of preterm infants,” she said. “In addition to the significant reduction in infections, there were no signs of intolerance or adverse reactions to the antimicrobial catheters.”
Informing physicians
Clinicians also struggle to follow good practice protocol when central lines are placed in areas that are challenging to maintain successfully.
“For example, keeping a high intra-jugular (IJ) dressing clean, dry and intact for seven days on a male patient with facial hair and a short neck is nearly impossible,” said Chapman. “One way to address this challenge is to educate physicians on optimum placement of central lines which takes into consideration the patient’s clinical need/condition and decreasing the patient’s risk for CLABSI. Assisting physicians to understand that the inserter takes care of that line for a matter of minutes, nursing has to care for that line for days — let’s give them the best chance to keep that line/dressing clean, dry and intact.”
Driving it home with data analysis
According to APIC recommendations, for every 250 hospital beds a facility has there should be one full-time infection preventionist. Unfortunately, according to research mentioned by Tammy Alvarado, RN, Business Leader, Infection Prevention Division, Q-Centrix, that’s not how it is at more than 50 percent of acute care facilities. Investing in data management technology designed specifically to track infection trends can be a huge help.
“When freed from having to collect, manage, and report data, hospital infection prevention staff and clinicians can focus on patient care and implementing the necessary changes to prevent CAUTI/CLABSI/VAPs,” said Alvarado. “Our solution offers the ability to attribute a range of factors to an infection, such as hospital bed and physician, that aid in data-driven decision-making. Overall, the data is best used for planning and implementing improvement efforts and measuring their effectiveness. This information drives various inquiries, such as root-cause and process failure analyses, that zero in on barriers to effective infection control.”
Alvaro says the technology is used at hundreds of facilities to help discover non-compliance such as inadequate care and attention to urethral areas and improper catheter securement, for example.
“Identifying these kinds of issues can lead to changes that ensure patient safety, such as improved care protocols and even customized alerts to speed up interventions,” Alvarado said. “For instance, having an IP present to enforce hospital policies on best practices for periurethral care may reduce CAUTI rates. If such policies do not exist, the IP can initiate efforts to develop and implement them. An IP may also support assessing whether catheter use is for convenience or medically necessary. This can reduce overuse that leads to unnecessary risk for both CAUTI and CLABSI. The same idea can be applied to ventilator use and reducing risk for VAP. Our solutions truly empower hospital IPs to be change agents. Q-Apps not only makes this possible, it takes it a step further by centralizing and standardizing the process for reporting infection data to public health agencies.”
References
- Novel Dual-balloon Urinary Catheters Reduce Catheter-associated Urinary Tract Infections; Department of Urology, University of South Florida, Tampa, FL; Tampa General Hospital, Tampa, FL; Kaiser Permanente Riverside Medical Center, Riverside, CA.
- Bacterial Biofilms and catheters: A key to understanding bacterial strategies in catheter-associated urinary tract infection; Can J Infect Dis Vol 3 No 5 September/October 1992.
- O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2011;39:S1—S34.
- Menyhay SZ, Maki DG. Disinfection of needleless catheter connectors and access ports with alcohol may not prevent microbial entry: the promise of a novel antiseptic-barrier cap. Infect Control Hosp Epidemiol 2006;27(1):23—27.
- Nuckols, T. K., Keeler, E., Morton, S. C., Anderson, L., Doyle, B., Booth, M., … Shekelle, P. (2016). Economic Evaluation of Quality Improvement Interventions for Bloodstream Infections Related to Central Catheters. JAMA Internal Medicine, 176(12), 1843. doi:10.1001/jamainternmed.2016.6610.
- Marschall, J, et al. Strategies to Prevent Central Line-Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infect Control Hosp Epidemiol 2014;35(7):753-771.
Visit www.hpnonline.com/vap-chat/ for sidebars on VAP prevention.

Valerie J. Dimond | Managing Editor
Valerie J. Dimond was previously Managing Editor of Healthcare Purchasing News.